Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Low-melting mixtures based on choline ionic liquids†
Doris
Rengstl
,
Veronika
Fischer
and
Werner
Kunz
*
Institute of Physical and Theoretical Chemistry, University of Regensburg, D-93040 Regensburg, Germany. E-mail: Werner.Kunz@ur.de; Fax: +49 941 943 4532; Tel: +49 941 943 4044
First published on 11th September 2014
Abstract
In this article a strategy is proposed for the design of low toxic, room temperature liquid low-melting mixtures (LMMs) which are entirely composed of natural materials. From literature it is well known that, in general, deep eutectic solvents based on choline chloride and dicarboxylic acids are LMMs, but not liquids at room temperature, with one exception: a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar mixture of malonic acid and choline chloride. Therefore, the starting point of this study was the decrease of the melting point of one of the components, namely the dicarboxylic acid, which is succinic, glutaric or adipic acid. For this purpose, one of the two protons of the acidic group was exchanged by a bulky unsymmetrical choline cation. The resulting ionic liquids (ILs) were still solid at room temperature, but have a reduced melting temperature compared to the corresponding acids. In the second step, mixtures of these ILs with choline chloride were prepared. It turned out that choline glutarate–choline chloride mixtures are liquids at room temperature at compositions containing 95–98 wt% of choline glutarate. Finally, urea was added as another hydrogen bond donor. Density, conductivity and viscosity measurements were performed for all obtained mixtures. Moreover, a Walden plot was drawn which indicates that all mixtures are liquids with fully dissociated ions moving independently. Therefore, they are considered as “good” ionic liquids and, thus, for example they can be used to exchange more toxic or less biodegradable ILs in application processes. A brief outlook containing application possibilities is given. It is demonstrated that choline dodecylsulfate is readily soluble in these mixtures, forming aggregates in the LMM at temperatures exceeding 55 °C.
1 molar mixture of malonic acid and choline chloride. Therefore, the starting point of this study was the decrease of the melting point of one of the components, namely the dicarboxylic acid, which is succinic, glutaric or adipic acid. For this purpose, one of the two protons of the acidic group was exchanged by a bulky unsymmetrical choline cation. The resulting ionic liquids (ILs) were still solid at room temperature, but have a reduced melting temperature compared to the corresponding acids. In the second step, mixtures of these ILs with choline chloride were prepared. It turned out that choline glutarate–choline chloride mixtures are liquids at room temperature at compositions containing 95–98 wt% of choline glutarate. Finally, urea was added as another hydrogen bond donor. Density, conductivity and viscosity measurements were performed for all obtained mixtures. Moreover, a Walden plot was drawn which indicates that all mixtures are liquids with fully dissociated ions moving independently. Therefore, they are considered as “good” ionic liquids and, thus, for example they can be used to exchange more toxic or less biodegradable ILs in application processes. A brief outlook containing application possibilities is given. It is demonstrated that choline dodecylsulfate is readily soluble in these mixtures, forming aggregates in the LMM at temperatures exceeding 55 °C.
Introduction
Interesting deep eutectic solvents (DESs) and low-melting mixtures (LMMs) are obtained by mixing two or three cheap, biodegradable and low toxic solids (sometimes also a liquid and a solid) to form a new liquid phase with a melting point lower than the melting points of the single components.1–7 This liquid phase is generated by strong and particular association of the substances through hydrogen bonds.1,4,5 The main advantages of DESs can be found in the easy tuning of their physico-chemical properties by simply changing either the components involved or the applied mixing ratio.1,4,5 They are promising new liquids to replace toxic ionic liquids (ILs) or common organic solvents in applications, e.g. in pharmaceutical formulations,8 dissolution or treatment of biomass,3,9–11etc.1,2,12The first deep eutectic mixture without a metal salt mentioned in the literature was a blend of choline chloride and urea in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 The freezing point of this mixture was determined to be 12 °C, being substantially lower than the melting points of both pure substances (urea: 133 °C and choline chloride: 302 °C). From these experiments it was deduced that hydrogen bonds, being formed between urea and the chloride anion, are mainly responsible for the observed decrease of the freezing point.5 In addition, mixtures of thiourea with oxalate anions show the same behavior.13 Conductivity and viscosity data of choline chloride with urea reveal that the choline chloride is completely dissociated and the ions move independently.4,5 Due to these advantageous properties, the use of such mixtures opens the possibility of replacing toxic imidazolium ionic liquids by more sustainable compounds.1,5
2.5 The freezing point of this mixture was determined to be 12 °C, being substantially lower than the melting points of both pure substances (urea: 133 °C and choline chloride: 302 °C). From these experiments it was deduced that hydrogen bonds, being formed between urea and the chloride anion, are mainly responsible for the observed decrease of the freezing point.5 In addition, mixtures of thiourea with oxalate anions show the same behavior.13 Conductivity and viscosity data of choline chloride with urea reveal that the choline chloride is completely dissociated and the ions move independently.4,5 Due to these advantageous properties, the use of such mixtures opens the possibility of replacing toxic imidazolium ionic liquids by more sustainable compounds.1,5
Another interesting work focuses on the formation of (highly viscous) DESs from dicarboxylic acids and choline chloride.4 As for all DESs, the fluidity was found to be linked to the size of the mobile species as well as to the size of the holes allowing the mobility.4 Further, analysis unveiled that one chloride ion is complexed by two carboxylic acids, resulting in the delocalization of charge and, thus, in a depression of the freezing point.4 However, apart from equimolar mixtures of choline chloride and malonic acid, most DESs containing choline chloride and dicarboxylic acids are solid at room temperature.4
As a consequence, a novel strategy is applied in the present work to take a step forward towards new, room temperature liquid DESs or LMMs composed of natural products.
In the first step, one proton of the used dicarboxylic acids (succinic, glutaric and adipic acid) was exchanged by a bulky choline cation in order to lower the melting point of the acidic component in analogy to choline carboxylates.14,15 In this way, indeed ILs were successfully generated. However, their melting points were still above room temperature. In order to further decrease the melting point of the choline dicarboxylates and to destroy the hydrogen bond network, choline chloride was added. Moreover, the influence of the addition of urea, a strong hydrogen bond donor, to the mixtures was investigated. The density, viscosity and conductivity behaviours were studied.
The components of the LMMs are low toxic and of biological origin. Choline chloride is biocompatible and known as a former vitamin B4. It has some important key functions in the human body, e.g. as a precursor for phospholipids and acetylcholine.16 Further, glutaric acid is contained in natural food products and fruits. It also has a high bacteriostatic activity and is metabolized very rapidly in the human body.17 Urea is highly water soluble and not toxic to the human body. It is produced in the body in mammalian metabolism and even salvaged due to the metabolic activity of the colonic microflora and, thus, further used in the body. On the other hand, it can be easily excreted in the urine.18
Choline dicarboxylate ILs, namely choline succinate (ChdiC4), choline glutarate (ChdiC5), choline adipate (ChdiC6), and the above mentioned LMMs were characterized by thermogravimetric and differential scanning calorimetric measurements. The temperature dependent viscosities, conductivities and densities of all prepared LMMs were measured in the temperature range between 25 and 85 °C. Further, a Walden plot was drawn to compare the produced “ILs” with classical ones.
Finally, three different choline containing surfactants (choline dodecylsulfate, hexadecylsulfate and oleate) were solubilised in the LMMs in order to check potential structuring by means of small and wide angle X-ray scattering experiments.
Experimental
Chemicals
Adipic acid, glutaric acid and succinic acid were all purchased from Alfa Aesar and had a purity of ≥99%. Choline chloride (ChCl) (purity ≥ 98%, Sigma Aldrich), urea (molecular biology grade, Serva) and 80 wt% aqueous choline bicarbonate solution (Sigma Aldrich, stored at 2 °C to avoid decomposition and without stabilizer) were used.Synthesis
Choline dicarboxylates (ChdiCm) with m = 4 (succinate), 5 (glutarate), and 6 (adipate) were synthesized according to the synthesis route of Petkovic et al. with minor modifications.19 To the equimolar amount of dicarboxylic acid, aqueous choline bicarbonate solution was added dropwise. In contrast to the synthesis of Petkovic et al.,19 the IL was lyophilized and then dried for more than two weeks using a high vacuum pump. No heating was done during this procedure to avoid decomposition of the choline cation.LMMs were prepared in a glove box under a dry nitrogen flow atmosphere to exclude contamination with traces of water stemming from air humidity. Four LMMs with different compositions were prepared. Compositions and abbreviations can be seen in Table 1. The mixtures were stirred for 24 hours at 60 °C until a viscous clear liquid was obtained. Subsequently, all mixtures were post-dried for one week in a high vacuum. The water content is listed in the ESI.†
| Abbreviation | ChdiC5 in wt% | ChCl in wt% | Urea in wt% |
|---|---|---|---|
| LMM1 | 96.00 | 4.00 | — |
| LMM1Urea | 92.80 | 3.87 | 3.33 |
| LMM2 | 98.00 | 2.00 | — |
| LMM2Urea | 96.34 | 1.97 | 1.69 |
Only LMMs containing choline glutarate were investigated in the frame of this work because from pretests it was observed that the mixtures of choline glutarate–choline chloride with 95 wt% to 98 wt% of choline glutarate are liquid at room temperature and possess the lowest freezing points compared to other compositions. Further, small amounts of urea were used. Larger quantities of urea were not solved completely. The formulae of all components are given in Fig. 1.
Thermogravimetric analysis (TGA)
The decomposition temperatures (Tdec) of the ILs, ChdiCm with m = 4, 5, 6, and the LMMs were measured by means of a thermogravimetric analyzer (TGA7, Perkin-Elmer) in a temperature range of 30 to 300 °C and 30 to 400 °C, respectively. All measurements were performed under constant nitrogen flow and at a heating rate of 10 K min−1. Decomposition temperatures were determined from the onset of mass loss derived from the intersection of the baseline before thermal decomposition with the tangent during mass loss.Differential scanning calorimetry (DSC)
Melting points, glass transition temperatures Tg as well as freezing points Tf of the ILs, ChdiCm with m = 4, 5, 6, and LMMs were analyzed by means of differential scanning calorimetry using a DSC30 (Mettler) in a three-cycle mode, at a heating rate of 1 K min−1. Samples were prepared in a glove box under a nitrogen atmosphere and sealed in aluminum pans. Measurements were performed under a nitrogen atmosphere by continuously flushing the instrument with nitrogen to avoid contamination with water. Examined temperature ranges were −80 to 95 °C and −80 to 25 °C for ILs and LMMs, respectively. In the case of the LMMs, no glass temperatures or freezing points were determinable within the examined temperature range. Therefore, attempts were made to manually evaluate the crystallization points of the LMMs by continuously cooling down the four different LMMs from 25 to 4 °C at a heating rate of 1 °C per 30 minutes before subsequent storage at −18 °C for several days.Density
Densities (ρ) of LMM1, LMM1Urea, LMM2, and LMM2Urea were determined from 25 to 85 °C by using a vibrating tube densimeter (DMA 5000M, Anton Paar). Received densities were used for the calculation of molar concentrations and molar volumes (Vm), necessary for the determination of the equivalent conductivity Λm. The uncertainty was calculated to be ±0.0001 g cm−3.Conductivity
Temperature dependent specific conductivities κ were measured at different temperatures from 25 to 85 °C, using a custom-designed apparatus, composed of a precision thermostat, a sine generator, a symmetrical Wheatstone bridge with Wagner earth and a resistance decade.20,21 Temperature control was achieved using a combination of a homebuilt precision thermostat and a commercial thermostat (Julabo FP40), yielding a temperature stability of ±0.01 °C. Samples were stored under a nitrogen atmosphere in capillary cells, each of them containing a three-electrode setup. The cell constant α was 34 cm−1. The electrical resistance was recorded at frequencies ranging from 100 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz. To eliminate disturbing effects caused by electrode polarization, the resistance R was extrapolated to R∞ = limν→∞R(ν).20 Specific conductivities were calculated according to κ = α/R∞. The temperature dependence of the cell constant was determined to be negligible22 and the uncertainty was estimated to be <1%.
000 Hz. To eliminate disturbing effects caused by electrode polarization, the resistance R was extrapolated to R∞ = limν→∞R(ν).20 Specific conductivities were calculated according to κ = α/R∞. The temperature dependence of the cell constant was determined to be negligible22 and the uncertainty was estimated to be <1%.
Viscosity
Temperature dependent viscosities η were measured using a Bohlin rheometer (CVO 120 High Resolution) with a plate/plate geometry (P20mm). The instrument was equipped with a temperature control unit, allowing the investigation at different temperatures (25 to 85 °C). Shear rates were varied between 0.00375 s−1 and 262 s−1. Results show that all LMMs are Newtonian fluids, having a constant shear stress to shear rate behaviour at all examined temperatures. In addition, all experiments were performed under an argon atmosphere. The uncertainty of about 1% was taken into account and the instrument was calibrated with calibration oil recommended by Bohlin instruments.Small and wide angle X-ray scattering (SAXS/WAXS)
X-ray scattering measurements were performed at the Institut de Chimie Séparative de Marcoule (France). The X-ray radiation was generated by a sealed molybdenum tube with a wavelength of λ = 0.71 Å. The tube was mounted on a bench built by XENOCS. A large two-dimensional automatic image plate system (MAR Research 345, diameter: 345 mm) is used for the scattered beam detection. The pixel resolution of the system was 150 × 150 μm. The different samples were assembled in 3 mm thick aluminium cells, which were sealed with Kapton foil of 25 μm thickness. The measurements were conducted at temperatures ranging between 25 and 70 °C with ΔT = ±2 °C. Two-dimensional spectra were integrated with the FIT2D software. Azymuthal integration was performed to obtain the scattering intensity as a function of the scattering vector q (= (4π/λ)·sinθ/2; with 2θ being the scattering angle). Acquisition time for each sample was 3600 seconds. Further, 2 wt% of the surfactant in LMM1Urea was investigated. Choline dodecylsulfate, choline hexadecylsulfate and choline oleate were used as surfactants. To obtain the absolute intensity of the spectra of LMM1Urea, the intensity of the empty cell was subtracted from the LMM1Urea spectra. To get absolute intensities of the solutions containing the surfactant, the scattering contributions of the empty cell and of the pure solvent LMM1Urea were subtracted from the spectrum of 2 wt% surfactant in LMM1Urea, taking into account the transmission factors and volume fraction.Results and discussion
Decomposition and melting/crystallization temperatures
The thermal decomposition of pure choline dicarboxylates, ChdiCm with m = 4, 5, 6, is a single step decomposition. Increasing decomposition temperatures were observed with increasing chain lengths, however, not showing any linear correlation. The observed decomposition temperatures (Tdec) of the LMMs are altogether above those of the pure ILs. While thermal decomposition of the LMMs not containing urea proceeds in a singular step, a two-step mechanism is observed for LMMs prepared with urea. In the latter case, the decomposition of urea takes place in the temperature range between around 160 and 250 °C according to the literature,23,24 while LMMs start to decompose at the temperature given in Table 2. The thermograms are shown in the ESI.†| Compound | T dec/°C |
|---|---|
| ChdiC4 | 242.9 |
| ChdiC5 | 243.3 |
| ChdiC6 | 245.2 |
| LMM1 | 268.7 |
| LMM1Urea | 282.4 |
| LMM2 | 273.0 |
| LMM2Urea | 280.3 |
Melting points of succinic, glutaric and adipic acid are 185 °C, 97.5 °C and 153.5 °C, respectively.25 The DSC measurements show that the choline cation is capable of lowering the melting temperatures of the choline dicarboxylates, ChdiCm with m = 4, 5, 6. The melting point is 61.2 ± 0.7 °C for ChdiC4, 39.3 ± 0 °C for ChdiC5 and 85.2 ± 0.7 °C for ChdiC6, respectively. It is assumed that the bulky and unsymmetrical structure of the choline cation hinders the arrangement of regular packing and, thus, lowers the melting temperatures of the choline dicarboxylates.
It is known that hydrogen bonds between the organic salt and the hydrogen bond donor cause charge delocalization and depression of the melting point.4,5 In this work, we take advantage of this phenomenon for the synthesis of LMMs. Choline glutarate, serving as the hydrogen bond donor, forms a complex with chloride ions (LMM1 and LMM2). Charge is delocalized and results in a decrease of the melting point of the mixture compared to the pure substances. No stable, long-term liquid room temperature LMMs were observed for mixtures of choline succinate and choline adipate with choline chloride. In the frame of this work, the influence of urea, representing another type of hydrogen bond donor, was also tested. DSC measurements performed for the determination of glass and freezing temperatures at a heating rate of 1 K min−1 and a temperature range of −80 to 25 °C were not successful. The following experiments show that the heating and cooling rate of 1 K min−1 was too fast to start the crystallization process at −18 °C. During a manual and non-automatized investigation LMM1 remains liquid at temperatures of −18 °C for a period of 3 days; afterwards it tends to crystallize. Similar results were obtained for the LMM2 system, where crystallization commences after storage at −18 °C for 7–8 days. In contrast to these systems, LMM1Urea and LMM2Urea provide higher liquid phase stabilization. After eight weeks, crystallization occurred for LMM1Urea and LMM2Urea. From all recorded data it can be concluded that the addition of urea successfully delays the crystallization processes in the examined LMMs at −18 °C.
Density
As expected, all examined LMMs possess densities higher than water.1 In general, one should assume that densities of the DESs or LMMs tend to increase with increasing fractions of choline chloride. However, this assumption could not be confirmed for the urea-free LMMs, because the difference in the amounts of choline chloride used in LMM1 and LMM2 is too small.1 While the molar ratio between choline glutarate and choline chloride remains the same in both LMM1/LMM1Urea and LMM2/LMM2Urea systems, only urea was added in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for choline chloride/urea.5 Taking into account the hole theory, which is used to explain the conductivity behavior and packing in DESs,1,4 the average hole radius decreases by introducing urea to the mixtures, therefore, leading to a density increase.1 The results are depicted in Fig. 2.
2 for choline chloride/urea.5 Taking into account the hole theory, which is used to explain the conductivity behavior and packing in DESs,1,4 the average hole radius decreases by introducing urea to the mixtures, therefore, leading to a density increase.1 The results are depicted in Fig. 2.
Conductivity
Specific conductivities, measured for the four LMMs in the temperature range from 25 to 85 °C, were found to vary between 0.01370 mS cm−1 and 0.70226 mS cm−1, thus being in accordance with the conductivity values typically found for ILs.26However, compared to imidazolium ILs the conductivities of the presently studied systems are lower to some extent.27,28 Higher conductivities were also reported for DESs composed of dicarboxylic acid and choline chloride in different ratios4 as well as for mixtures of urea and choline chloride.1 However, obtained values agree well with results described for the choline oligoether carboxylate IL (Ch-TOTO).29
Specific conductivities of LMM1, LMM1Urea, LMM2, and LMM2Urea were found to be temperature dependent.
As seen in Fig. 3 a linear correlation exists between the natural logarithm of the specific conductivity κ and the reciprocal temperature. Consequently, the Arrhenius eqn (1)4,30,31 can be used as a fitting equation, but the Vogel–Fulcher–Tammann (VFT) eqn (2)14,32 is also suitable for the evaluation of the temperature dependent changes in conductivities:
 | (1) |
 | (2) |
 | ||
| Fig. 3 Plot of the natural logarithm of the specific conductivity κ of the LMMs versus the reciprocal temperature (Arrhenius fit R2 = 0.989 to 0.998). | ||
For the Arrhenius model a temperature independent activation energy of conductivity EΛ is assumed, while the Vogel–Fulcher–Tammann model proposes a temperature dependent activation energy EΛVFT.32T0κ represents the ideal glass temperature.14,32 However, both models are of empirical nature and can be used as fitting models for the purpose of this study. The Arrhenius model is thereby preferred because the introduction of a further variable in the fitting process (T0κ) seems to be unnecessary. Nevertheless, both models were applied to allow comparison of the data with the choline oligoether IL reported previously.14
Values obtained from the two fittings for the activation energies are shown in the ESI.† The activation energy of the conductivity does not depend on the small amount of choline chloride or urea used in the LMMs, and measured conductivities of all compositions were basically the same. Activation energies are comparable with the one observed for the deep eutectic mixture of succinic acid and choline chloride (EΛ = 54.3 ± 4.1 kJ mol−1).4 The activation energies of malonic acid (EΛ = 29.0 ± 1.2 kJ mol−1) and oxalic acid (EΛ = 34.6 ± 1.5 kJ mol−1) with choline chloride are lower according to the smaller size of the molecules and increasing charge per molecule.4 Also the choline oligoether IL Ch-TOTO shows a much smaller activation energy EΛVFT = 8.7 ± 0.1 kJ mol−1 as found here.29
Viscosity
Temperature dependent viscosities, obtained for the LMMs from dynamic viscosity measurements, shown in Fig. 4, are influenced by the amount of choline chloride as well as by the amount of urea in the mixtures. Viscosities of the LMMs were found to significantly increase with increasing quantities of hydrogen bonds present in the mixtures.1 | ||
| Fig. 4 Plot of the natural logarithm of the viscosity η of the four LMMs versus the reciprocal temperature (Arrhenius fit R2 = 0.989 to 0.998). | ||
As a consequence, the viscosity decreases with increasing amounts of choline chloride. In addition, a further increase in viscosity is observed when urea, representing another hydrogen bond donor, is added to the system. In general, viscosity changes are quite small between the different mixtures due to the small changes in the ratios between the different components in the LMMs.
Walden plot
The interplay between the molar conductivity, also represented by the ion mobility, and the fluidity, reciprocal viscosity, can be seen in the Walden plot (see Fig. 5). | ||
| Fig. 5 Walden plot, comparing the LMMs at different temperatures (25 to 85 °C) with the ideal line for 1 M KCl. | ||
The Walden plot is a useful tool to compare ILs with the LMMs and to determine the ion association.4 The Walden plot was used by Angell and coworkers to characterize ILs according to their degree of ionicity.33–35 They used this plot to categorize ILs as “good” or “poor” ionic liquids, “superionic” liquids and so on.34 The theory is based on Walden's observation27,29 that the equivalent conductivity of a strong electrolyte in aqueous solution is inversely proportional to the viscosity. The equivalent conductivity and inverse viscosity are influenced by temperature in the same way.27,29 According to Angell et al. it is possible to give a statement about the cation and anion association by the use of the Walden rule.33,36 The black line in Fig. 5 has a slope of 1 and marks the region of fully dissociated salts like a dilute solution of 1 M KCl.26,27 This means that ions in solution are able to move independently of their ambient ions. Angell et al. introduced the ΔW value, the vertical deviation to this ideal line, to characterize ILs according to this value. In this context, “good” ILs are fully dissociated and show a ΔW < 1. ILs with ΔW = 1 exhibit only 10% of the ionic conductivity as would have been expected at the ideal line of 1 M KCl.28
Points depicted in Fig. 5 represent the temperature dependent molar conductivities and fluidities of LMM1, LMM2, LMM1Urea and LMM2Urea, being visibly very close to the ideal line of the Walden plot. All points show a vertical deviation which is smaller than 0.25. Consequently, choline chloride and choline glutarate are fully dissociated in the LMMs and behave like “good” ILs, and none or only a few ion pairs are expected to exist in the examined mixtures.
However, we are aware that this plot does not allow us to draw a quantitative conclusion about ion dissociation. To do this, the best way would be to measure independently diffusion coefficients. An alternative has been proposed by MacFarlane et al. by also considering the ionic radii.35 We generated them using ChemDraw and made a new corrected Walden plot in line with the suggestion by MacFarlane et al. As can be seen in Fig. 6, this plot leads to significantly lower points suggesting that at least the urea-free mixtures are partly associated.
 | ||
| Fig. 6 Modified Walden plot, taking into account the differences in ion radii.35 | ||
Surprisingly, the addition of urea slightly increased electric conductivity. This may hint at a different charge transport, perhaps proton hopping involving urea molecules or simply urea increasing ion dissociation by specific interactions. However, for the moment this remains speculation and even a detailed MD simulation would not deliver an unambiguous answer. In our opinion, only the determination of the urea dissociation constant would help us to check this possibility. However, this is a difficult task in such complex and highly charged systems and out of the scope of the present work.
X-ray characterisation
The system of LMM1Urea with and without surfactant was analysed with X-ray scattering experiments. The results are depicted in Fig. 7 and 8.No temperature dependent behaviour of the SAXS and WAXS spectra was found and also no self-structuring of the pure LMM was observed. This observation is in good agreement with the assumption that DESs and LMMs are non-volatile and show very small isothermal compressibilities. This can be assumed taking into account the absolute intensity at q = 0 and bearing in mind that the absolute intensity I(q = 0) is directly proportional to the temperature T and the isothermal compressibility χT (I(q = 0) ∼ T·χT).37
Dissolution of choline surfactants in LMMs
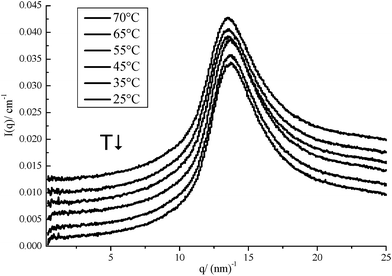
Having prepared “green” DESs and LMMs, several questions arise. Is structuring possible in these mixtures? Can they be used to dissolve biomass, especially biopolymers? Is it possible to use them in formulation? Dissolution of cellulose was found to be impossible, as it seems that the hydroxyl groups of choline are linked to cellulose due to hydrogen bonds, thus stabilizing the cellulose system.38A promising formulation could be the dissolution of choline surfactants in DESs or LMMs. Therefore, 2 wt% of choline dodecylsulfate, hexadecylsulfate or oleate was dissolved in the four examined LMMs. At room temperature, surfactant crystals remain solid in the observed systems. Upon heating to 50 °C, the mixtures containing 2 wt% choline dodecylsulfate became transparent and no birefringence was observed during microscopical analysis with crossed polarisers. In contrast, no complete dissolution of the surfactant was observed at temperatures up to 90 °C for mixtures containing 2 wt% surfactant either choline oleate or choline hexadecylsulfate.
As shown in Fig. 8, weak reflections are found in the WAXS region and prove the existence of a crystalline substance at temperatures below 50 °C. In the SAXS region, a defined peak at 3.07 nm−1 was observed. This can be due to a d-spacing relative to the alkyl chain length of the surfactant, which, according to Tanford, has a chain length of 17 Å.39
The SAXS spectra of 2 wt% choline hexadecylsulfate and choline oleate in LMM1Urea (not shown here) showed defined reflections at 2.51 nm−1 or 1.51 nm−1, resulting from the alkyl chains of the surfactants. Obviously, only choline dodecylsulfate dissolves sufficiently and is capable of forming aggregates in LMM1Urea.
To evaluate the size and shape of the aggregates, higher scattering intensities and even lower q values are necessary. Therefore, the use of synchrotron radiation is essential for the analysis of this system. In summary, it was nevertheless demonstrated that formation of aggregates is possible in these LMMs.
Conclusion
A two-step strategy to form green room temperature liquids containing low toxic dicarboxylic acids is presented within this work. In the first step, hydrogen bond networks, usually existing in systems of pure dicarboxylic acids, are simply destroyed by quantitatively exchanging protons of one carboxylic group by choline cations, resulting in significantly lowered melting points. The remaining second protonated carboxylic group is still capable of forming hydrogen bonds, thus leading to a delocalization of the charge in the examined mixtures. In the second step, the choline dicarboxylate IL was mixed with choline chloride to destroy the hydrogen bond network in the choline dicarboxylate.This two-step strategy towards “green” LMMs was found to work especially well when using glutaric acid resulting in a highly viscous (but liquid) LMMs at room temperature. The disadvantages of the LMMs synthesized within the frame of this work are their observed high viscosities and low conductivities. In addition, it was demonstrated within this study that the addition of a second hydrogen bond donor (urea) also has a strong influence on the viscosity of choline glutarate–choline chloride LMMs. Increasing viscosities were observed upon increasing amounts of the second hydrogen bond donor. Partly associated anions and cations were observed in the examined LMMs, at least in the mixtures without urea, as inferred from the modified Walden plot. It was further demonstrated that choline dodecylsulfate is capable of forming aggregates in LMM1Urea.
In view of possible applications for the examined LMMs, observed high viscosities of these mixtures strongly limit their potential suitability for electrochemical applications. On the other hand, one might indeed think of their potential use in formulations for pharmaceutical issues, as the examined systems are advantageous in terms of their easy preparation. Even the ILs show an easy and cheap synthesis route. Their non-toxicity and their biological origin further allow easy decomposition by the human body. One possible application even due to their low oral toxicity could be their use as carriers in pharmacokinetic studies on mice or rats to increase the admittance of scarcely soluble substances in water. This application was already approved as possible for choline chloride–urea mixtures and mixtures of malonic acid with choline chloride.8
Acknowledgements
We thank Dr Olivier Diat for making the X-ray scattering experiments available in his lab at the Institut de Chimie Séparative de Marcoule (France). Further, we thank Prof. Richard Buchner, Dr Thomas Sonnleitner and Dr Rainer Müller for their technical support.Notes and references
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- C. Russ and B. Konig, Green Chem., 2012, 14, 2969–2982 RSC.
- M. Francisco, A. van den Bruinhorst and M. C. Kroon, Angew. Chem., Int. Ed., 2013, 52, 3074–3085 CrossRef CAS PubMed.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- A. P. Abbott, R. C. Harris and K. S. Ryder, J. Phys. Chem. B, 2007, 111, 4910–4913 CrossRef CAS PubMed.
- V. Fischer and W. Kunz, Mol. Phys., 2014, 112, 1241–1245 CrossRef CAS.
- H. G. Morrison, C. C. Sun and S. Neervannan, Int. J. Pharm., 2009, 378, 136–139 CrossRef CAS PubMed.
- H. Garcia, R. Ferreira, M. Petkovic, J. L. Ferguson, M. C. Leitao, H. Q. N. Gunaratne, K. R. Seddon, L. P. N. Rebelo and C. Silva Pereira, Green Chem., 2010, 12, 367–369 RSC.
- T. V. Doherty, M. Mora-Pale, S. E. Foley, R. J. Linhardt and J. S. Dordick, Green Chem., 2010, 12, 1967–1975 RSC.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- M. Francisco, A. van den Bruinhorst and M. C. Kroon, Angew. Chem., 2013, 125, 3152–3163 CrossRef.
- S. Saito, M. Lee and W.-Y. Wen, J. Am. Chem. Soc., 1966, 88, 5107–5112 CrossRef CAS.
- R. Klein, H. Dutton, O. Diat, G. J. T. Tiddy and W. Kunz, J. Phys. Chem. B, 2011, 115, 3838–3847 CrossRef CAS PubMed.
- R. Klein, D. Touraud and W. Kunz, Green Chem., 2008, 10, 433–435 RSC.
- J. K. Blusztajn, Science, 1998, 281, 794–795 CrossRef CAS.
- H. L. Merten and G. L. Bachman, J. Food Sci., 1976, 41, 463–464 CrossRef CAS PubMed.
- A. Jackson, Arch. Dis. Child., 1994, 70, 3 CrossRef CAS.
- M. Petkovic, J. L. Ferguson, H. Q. N. Gunaratne, R. Ferreira, M. C. Leitao, K. R. Seddon, L. P. N. Rebelo and C. S. Pereira, Green Chem., 2010, 12, 643–649 RSC.
- J. Barthel, F. Feuerlein, R. Neueder and R. Wachter, J. Solution Chem., 1980, 9, 209–219 CrossRef CAS.
- J. Barthel, R. Wachter and H. J. Gores, in Modern Aspects of Electrochemistry, ed. B. E. Conway and J. O. M. Bockris, Springer, US, 1979, ch. 1, pp. 1–79 Search PubMed.
- R. A. Robinson and R. H. Stokes, Electrolyte solutions, Butterworths, London, 1959 Search PubMed.
- A. M. Wynne, J. Chem. Educ., 1987, 64, 180 CrossRef CAS.
- P. M. Schaber, J. Colson, S. Higgins, D. Thielen, B. Anspach and J. Brauer, Thermochim. Acta, 2004, 424, 131–142 CrossRef CAS PubMed.
- D. J. Hanahan and D. M. Small, The Physical Chemistry of Lipids: From Alkanes to Phospholipids, Plenum Press, 1986 Search PubMed.
- O. Zech, M. Kellermeier, S. Thomaier, E. Maurer, R. Klein, C. Schreiner and W. Kunz, Chem. – Eur. J., 2009, 15, 1341–1345 CrossRef CAS PubMed.
- O. Zech, A. Stoppa, R. Buchner and W. Kunz, J. Chem. Eng. Data, 2010, 55, 1774–1778 CrossRef CAS.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS.
- R. Klein, O. Zech, E. Maurer, M. Kellermeier and W. Kunz, J. Phys. Chem. B, 2011, 115, 8961–8969 CrossRef CAS PubMed.
- A. P. Abbott, J. C. Barron, K. S. Ryder and D. Wilson, Chem. – Eur. J., 2007, 13, 6495–6501 CrossRef CAS PubMed.
- J. O. M. Bockris and A. K. N. Reddy, Modern Electrochemistry; an Introduction to an Interdisciplinary Area, 1970 Search PubMed.
- A. Grandjean, M. Malki, C. Simonnet, D. Manara and B. Penelon, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 054112 CrossRef.
- M. Yoshizawa, W. Xu and C. A. Angell, J. Am. Chem. Soc., 2003, 125, 15411–15419 CrossRef CAS PubMed.
- W. Xu, E. I. Cooper and C. A. Angell, J. Phys. Chem. B, 2003, 107, 6170–6178 CrossRef CAS.
- D. R. MacFarlane, M. Forsyth, E. I. Izgorodina, A. P. Abbott, G. Annat and K. Fraser, Phys. Chem. Chem. Phys., 2009, 11, 4962–4967 RSC.
- P. Walden, Z. Phys. Chem., Stoechiom. Verwandtschaftsl., 1906, 55, 207–249 CAS.
- D. Orthaber, A. Bergmann and O. Glatter, J. Appl. Crystallogr., 2000, 33, 218–225 CrossRef CAS.
- A. P. Abbott, T. J. Bell, S. Handa and B. Stoddart, Green Chem., 2006, 8, 784–786 RSC.
- C. Tanford, J. Phys. Chem., 1972, 76, 3020–3024 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cp02860k |
| This journal is © the Owner Societies 2014 |