Electronic effect of terminal acceptor groups on different organic donor–acceptor small-molecule based memory devices†
Abstract
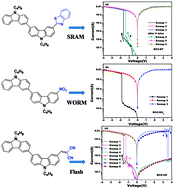
In this work, three new organic donor–acceptor small-molecules, in which bicarbazole served as the electron donor, and benzothiazole, nitryl or 1,1′-dicyanovinyl were used as the electron acceptor, were designed and synthesized in order to fabricate sandwiched memory devices. Acceptors with a variable electron-delocalized extent and electron-withdrawing strength were attached to the molecular backbone in order to investigate the effect on the devices switching behavior. The bi-n-butylcarbazole benzothiazole (BCZ-BT) based memory device exhibited volatile static random access memory (SRAM) switching behaviour, while the devices based on bi-n-butylcarbazole nitryl (BCZ-NO2) was found to exhibit stable nonvolatile write-once-read-many-times (WORM) data storage characteristics and the bi-n-butylcarbazole dicyanovinyl (BCZ-CN) device acted as rewritable flash memory with a higher ON/OFF current ratio of about 104. Therefore, tunable data storage devices synthesized by adjusting the terminal acceptor groups offer feasible guidance for the rational design of organic molecules to achieve superior memory performance.

 Please wait while we load your content...
Please wait while we load your content...