 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A new ultrafast superionic Li-conductor: ion dynamics in Li11Si2PS12 and comparison with other tetragonal LGPS-type electrolytes†
Alexander
Kuhn
a,
Oliver
Gerbig
a,
Changbao
Zhu
a,
Frank
Falkenberg
a,
Joachim
Maier
a and
Bettina V.
Lotsch
*abc
aMax Planck Institute for Solid State Research, Heisenbergstr. 1, 70569 Stuttgart, Germany. E-mail: a.kuhn@fkf.mpg.de; b.lotsch@fkf.mpg.de
bDepartment of Chemistry, Ludwig-Maximilians-Universität München, Butenandtstr. 5-13, 81377 München, Germany
cNanosystems Initiative Munich (NIM) and Center for Nanoscience (CeNS), Schellingstr. 4, 80799 München, Germany
First published on 30th May 2014
Abstract
We report on a new ultrafast solid electrolyte of the composition Li11Si2PS12, which exhibits a higher room-temperature Li ion diffusivity than the present record holder Li10GeP2S12. We discuss the high-pressure synthesis and ion dynamics of tetragonal Li11Si2PS12, and comparison is made with our investigations of related members of the LMePS family, i.e. electrolytes of the general formula Li11−xMe2−xP1+xS12 with Me = Ge, Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li10GeP2S12, Li7GePS8, Li10SnP2S12. The structure and dynamics were studied with multiple complementary techniques and the macroscopic diffusion could be traced back to fast Li ion hopping in the crystalline lattice. A clear correlation between the diffusivity and the unit cell volume of the LGPS-type electrolytes was observed.
Li10GeP2S12, Li7GePS8, Li10SnP2S12. The structure and dynamics were studied with multiple complementary techniques and the macroscopic diffusion could be traced back to fast Li ion hopping in the crystalline lattice. A clear correlation between the diffusivity and the unit cell volume of the LGPS-type electrolytes was observed.
In 2011, the new solid lithium electrolyte Li10GeP2S12 (LGPS) was reported, featuring liquid-like Li ion conduction in a crystalline solid matrix.1,2 The ultrafast room temperature transport of tetragonal LGPS with a conductivity of several mS cm−1 came as a surprise as it exceeds the values of the best crystalline Li conductors by one order of magnitude. Therefore, there has been a strong upsurge of interest recently in realizing LGPS-type materials based on the homologous elements Si and Sn. A theoretical study published by Ceder and coworkers highlights the potential of such hypothetical tetragonal LGPS-type Li ion conductors.3 Li10SnP2S12 was recently reported4,5 and showed slightly reduced Li mobility as compared to LGPS, in line with the predictions by Ceder et al.
Here, we (i) report the successful high-pressure synthesis of the Si-analogue, Li11Si2PS12, and (ii) present a comparative fundamental study of the Li ion dynamics in the four LGPS-type electrolytes reported so far, Li11Si2PS12 (LSiPS, this study), Li10SnP2S12 (LSnPS, this study and ref. 4 and 5), Li10GeP2S12 (ref. 1 and 6) and Li7GePS8 (ref. 6). LSnPS and LSiPS were comprehensively characterized both with respect to their structure and Li ion dynamics and compared with the results previously published on LGPS.6 In excellent agreement with the theoretical predictions in ref. 3, LSiPS shows an even higher Li diffusivity than LGPS while LSnPS has a slightly lower Li diffusivity. A clear correlation between the diffusivity and the unit cell volume was observed.
Tetragonal Li10SnP2S12 was prepared by heating elemental Sn, P, S, and Li2S to 653 K for 10 h with an additional sintering step at 723 K for two days, cf.ref. 4 and 5. The tendency of the larger SnIV to reside in six-fold rather than four-fold coordination (the latter being desired for LSnPS) is reflected by the presence of side phases with edge-sharing SnS6 building units (such as Li2SnS3) when stoichiometric amounts of the starting materials are used. A 10–20% excess of Li2S, however, completely prevents the formation of these layered side phases. It should be noted that we used a slight excess of S yielding approx. 1 atm S at the reaction conditions in order to ensure complete oxidation of Sn and P.
The preparation of phase-pure hypothetical tetragonal Li10SiP2S12 was not successful by means of a conventional solid-state approach. Instead, the main phase at all temperatures between 573 K and 1023 K was the orthorhombic modification of the LSiPS solid solution, which was known to possess distinctly less favorable transport properties.7 The fact that the tetragonal modification has a slightly higher density than the orthorhombic one (for LGPS, compare ref. 1 and 10), and that a higher Si content should enhance the stability owing to the lower ionic radius of Si led us to the successful preparation of Li11Si2PS12 by high-pressure synthesis (see ESI,† S1 for further details). We obtained almost phase pure Li11Si2PS12, which is referred to as LSiPS in the following.
Fig. 1a depicts the unit cell of the generic tetragonal LGPS structure as obtained from single-crystal X-ray diffraction of Li10GeP2S12.8 The structure contains PS4 and GeS4 tetrahedra which are charge-compensated by Li ions. In the tetragonal LGPS structure type, there exist two sets of tetrahedra with the central atom on a 4d and 2b site, respectively, with the tetrahedra around the 4d site being considerably larger. Consequently, in tetragonal LGPS, the 4d site is occupied by both Ge and P, while the smaller 2b site is solely occupied by P.1,8 The occupancy of the mixed occupied 4d site (GexP1−x) can be varied in the range of 0.5 ≤ x(Ge) ≤ 0.75 with the end members of the accessible range of the solid solution being roughly Li7GePS8 and Li10GeP2S12.6,9Fig. 1b shows the XRD patterns with single-phase Rietveld refinements for Li10SnP2S12 and Li11Si2PS12 in comparison with the patterns for tetragonal LGPS (Li7GePS8 and Li10GeP2S12). All samples show the desired tetragonal structure1,6,8 and are phase-pure on the XRD level except for a weak additional reflection for LSiPS (marked by an asterisk and tentatively ascribed to a high-pressure modification of S11). The cell parameters are listed in Table S1 (ESI†). According to the Rietveld refinement (see ESI,† S2), in Li10SnP2S12 the 4d site is occupied by Sn and P (x(Sn) = 0.47), whereas again, the 2b site is occupied by P only. This is in line with the results from single-crystal X-ray diffraction recently published by Bron et al.4 The accessible range of the LSnPS solid solution is much narrower than in case of LGPS – we obtained the tetragonal modification only for values of x(Sn) very close to 0.5. For other Sn/P ratios, two phases were obtained: tetragonal LSnPS of the composition Li10SnP2S12 and the orthorhombic modification as side phase. This can be explained by the larger ionic radius of SnIV as compared to GeIV, rendering a higher occupancy of this position relative to PV energetically unfavourable. The opposite is true for LSiPS: SiIV is only slightly larger than the isoelectronic PV ion. Therefore, in order to stabilize the tetragonal modification with two sets of differently-sized tetrahedra, the 4d site has to be occupied by Si to a much higher extent as compared to Ge or Sn. This explains why the tetragonal modification is obtained for the stoichiometry Li11Si2PS12 rather than Li10SiP2S12.
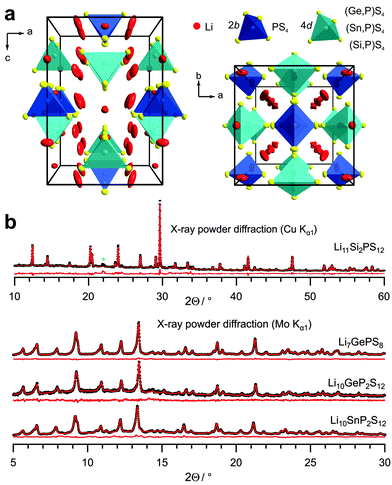 | ||
| Fig. 1 (a) Crystal structure of tetragonal LGPS as obtained from single-crystal X-ray diffraction.8 (b) X-ray powder diffraction and Rietveld refinement of Li11Si2PS12 and Li10SnP2S12 in comparison to previously reported Li10GeP2S12 and Li7GePS8.6 The side phase is marked by a green asterisk. | ||
31P MAS NMR was used in order to probe the relative amount of P residing on the 4d and 2b sites (cf.Fig. 1a). This is of special importance for the structural elucidation of the LSiPS sample because here, this information is not accessible from X-ray diffraction since SiIV and PV are isoelectronic. Fig. 2 shows the 31P MAS NMR spectra of the tetragonal LGPS-type electrolytes. The spectrum of Li10SnP2S12 is very similar to that of Li10GeP2S12: as expected from Rietveld refinement, the relative intensity of the signals assigned to the 4d and 2b sites is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 within 5% error. The third signal shows the chemical shift typical of the orthorhombic modification, which is, however, not observed in the X-ray patterns. Therefore, we assign it to the presence of a side phase of low crystallinity, which resembles the local structure of the orthorhombic modification (see also ref. 6). For Li11Si2PS12, the structural model assumed above – judging only from the stoichiometry of the sample – is largely verified. Only a small amount of P resides on the mixed-occupied 4d site (∼10%). Thus, the occupancy of the 4d site for all LGPS-type samples follows a clear trend. For the largest ion, Sn, the occupancy is close to x(Sn) ≈ 0.50. For Ge, the occupancy ranges from 0.5 < x(Ge) < 0.75, while for the small Si, x(Si) ≈ 0.95.
1 within 5% error. The third signal shows the chemical shift typical of the orthorhombic modification, which is, however, not observed in the X-ray patterns. Therefore, we assign it to the presence of a side phase of low crystallinity, which resembles the local structure of the orthorhombic modification (see also ref. 6). For Li11Si2PS12, the structural model assumed above – judging only from the stoichiometry of the sample – is largely verified. Only a small amount of P resides on the mixed-occupied 4d site (∼10%). Thus, the occupancy of the 4d site for all LGPS-type samples follows a clear trend. For the largest ion, Sn, the occupancy is close to x(Sn) ≈ 0.50. For Ge, the occupancy ranges from 0.5 < x(Ge) < 0.75, while for the small Si, x(Si) ≈ 0.95.
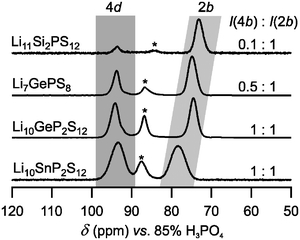 | ||
| Fig. 2 31P MAS NMR spectra (νrot = 12 kHz, B0 = 9.4 T) of the different isostructural materials crystallizing in the tetragonal LGPS-type. The spectra for Li7GePS8 and Li10GeP2S12 as well as the assignment of the lines were taken from ref. 6. The asterisks denote impurities with a chemical shift typical of the orthorhombic modification. | ||
In order to characterize the Li diffusivity in the LGPS-type materials Li10SnP2S12 and Li11Si2PS12, we performed 7Li PFG NMR measurements. The obtained Li tracer-diffusion coefficients Dtr (3D diffusion, cf.ref. 6) are shown in Fig. 3a in comparison with those previously reported for Li10GeP2S12.6 Note that in all cases, the measured diffusion coefficients can clearly be assigned to diffusion in the bulk of the tetragonal LGPS-type electrolytes, since the quadrupolar structure of the decaying NMR signal showed the finger-print (see Fig. 4a and b) of the tetragonal modification, which is distinct from that of orthorhombic or amorphous side phases. The diffusivity of Li10SnP2S12 is slightly lower than that of Li10GeP2S12 and the activation energy is slightly higher (0.23(1) eV vs. 0.21(1) eV). In contrast, the diffusivity of Li11Si2PS12 is even higher than that of Li10GeP2S12 with a slightly lower activation energy (0.19(1) eV vs. 0.21(1) eV). We would like to point out that this trend is – both qualitatively and quantitatively – in very good agreement with theoretical calculations by Ong et al.3 as presented in Table 1. Note that the theoretical diffusion coefficients taken from the MD simulation in ref. 3 have been extrapolated from 600 K down to room temperature in order to compare them with our experimental data. As shown in Fig. 3b, a clear correlation between the unit cell volume of the tetragonal LGPS-type materials and their diffusion parameters is observed. LSiPS shows the smallest unit cell volume and enhanced Li diffusivity, while LSnPS shows the largest unit cell volume and the lowest diffusivity.
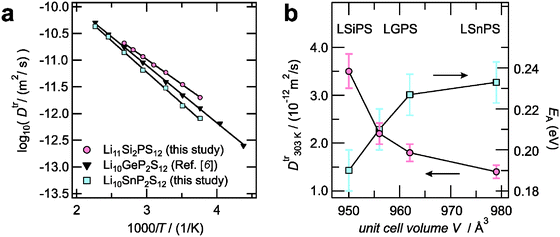 | ||
| Fig. 3 (a) Li tracer-diffusion coefficients of LGPS-type electrolytes as measured by 7Li PFG NMR. (b) Correlation between diffusion parameters and unit cell volume for LGPS-type electrolytes. | ||
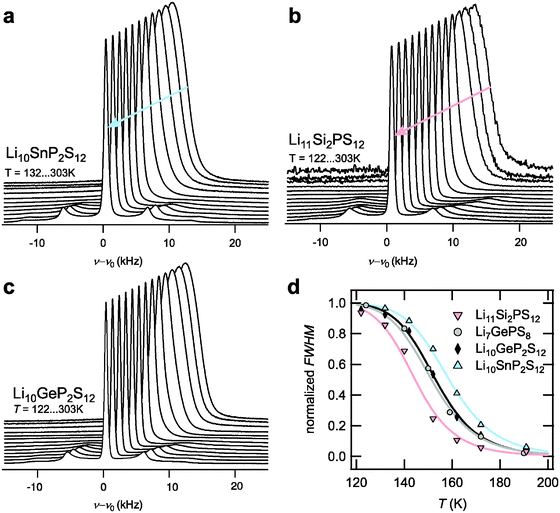 | ||
| Fig. 4 Temperature-dependent 7Li NMR spectra of (a) Li10SnP2S12, (b) Li11Si2PS12, and (c) Li10GeP2S12 (taken from ref. 6). (d) Comparison of the normalized narrowing curves of the LGPS-type electrolytes. | ||
| Compound | D th /m2 s−1 | D exp/m2 s−1 | E A,th/eV | E A,exp/eV |
|---|---|---|---|---|
| a This work. b From ref. 6. c Extrapolated from Fig. 3 in ref. 3. d From ref. 3. e From ref. 4. | ||||
| Li11Si2PS12 | — | 3.5 × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
— | 0.19(1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Li10SiP2S12 | 1.9 × 10−11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
— | 0.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
— |
| Li10GeP2S12 | 1.0 × 10−11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
2.2 × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
0.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
0.21(1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Li7GePS8 | — | 1.8 × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
— | 0.23(1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Li10SnP2S12 | 4.4 × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
1.4 × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
0.24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
0.23(1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Li10SnP2S12 | — | 1.8 × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
— | — |
While 7Li PFG NMR probes Li ion dynamics occurring on the micron scale and in the time scale of milliseconds, NMR relaxometry is sensitive to Li site-to-site hopping on the Ångström scale and in the time scale of microseconds (transversal relaxation) or nanoseconds (longitudinal relaxation).12 Thus, NMR relaxometry allows connecting the observed diffusion processes with their microscopic origin, i.e. site-to-site hopping of Li ions in the structure. Here, we measured the transversal relaxation – its Fourier transform being the static 7Li NMR spectrum – in order to probe the Li hopping processes. The temperature-dependent 7Li NMR spectra of LSnPS and LSiPS are displayed in Fig. 4a and b, respectively. As expected for isostructural compounds, the overall behavior of the line shape is very similar to that observed for LGPS6 (see Fig. 4c). At low temperatures, the central transition of the 7Li line is broad and Gaussian-shaped representing the static homonuclear and heteronuclear dipolar interaction of the 7Li spins with their environment. At higher temperatures, as the Li jump rate exceeds the time scale of the dipolar interactions determined by the rigid-lattice line width, the line narrows successively showing a sharp Lorentzian-shaped line at higher temperatures. From the onset of the motional narrowing at TMN, a jump rate can be assessed according to the narrowing condition 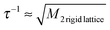 whereby M2
whereby M2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rigid
rigid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lattice is the second moment of the rigid lattice line.12 In Fig. 4d, the narrowing curves of the four LGPS-type electrolytes are compared. The trend already observed in PFG NMR is clearly reproduced. The Li jump rates at TMN amount to 1.5 × 104 s−1 @ 125 K for LSiPS, 1.4 × 104 s−1 @ 135 K for LGPS, and 1.4 × 104 s−1 @ 145 K for LSnPS, respectively (values included in Fig. 5d, see ESI,† S4 for further details). As already discussed for the LGPS samples in ref. 6, the distinct quadrupolar powder pattern, which is observed for both LSiPS and LSnPS at higher temperatures, represents the quadrupolar coupling between the quadrupolar moment of the 7Li spins and a mean electric field gradient they are exposed to while diffusing through the tetragonal lattice. As observed in the experiment, this mean electric field gradient retains the axial symmetry of the tetragonal structure (δQ = 23.5…25.9 kHz, ηQ = 0). Thus, the appearance of this distinct quadrupolar powder pattern indicates that the fast Li dynamics measured in fact occurs within crystallites of tetragonal LSnPS or LSiPS.
lattice is the second moment of the rigid lattice line.12 In Fig. 4d, the narrowing curves of the four LGPS-type electrolytes are compared. The trend already observed in PFG NMR is clearly reproduced. The Li jump rates at TMN amount to 1.5 × 104 s−1 @ 125 K for LSiPS, 1.4 × 104 s−1 @ 135 K for LGPS, and 1.4 × 104 s−1 @ 145 K for LSnPS, respectively (values included in Fig. 5d, see ESI,† S4 for further details). As already discussed for the LGPS samples in ref. 6, the distinct quadrupolar powder pattern, which is observed for both LSiPS and LSnPS at higher temperatures, represents the quadrupolar coupling between the quadrupolar moment of the 7Li spins and a mean electric field gradient they are exposed to while diffusing through the tetragonal lattice. As observed in the experiment, this mean electric field gradient retains the axial symmetry of the tetragonal structure (δQ = 23.5…25.9 kHz, ηQ = 0). Thus, the appearance of this distinct quadrupolar powder pattern indicates that the fast Li dynamics measured in fact occurs within crystallites of tetragonal LSnPS or LSiPS.
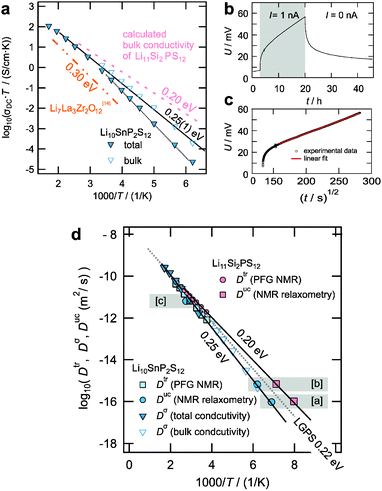 | ||
| Fig. 5 (a) Total and bulk conductivity of LSnPS as extracted from impedance spectroscopy measurements (see ESI,† for details). The expected bulk conductivity of LSiPS as calculated from NMR diffusivity data is included as a dotted line. For comparison, the dashed dotted line represents the conductivity of the best oxidic solid electrolyte, Li7La3Zr2O12. (b) Galvanostatic dc polarization measurement on a symmetric Au|LSnPS|Au cell at 473 K. (c) The polarization curve from (b), as a function of t1/2, with linear fit. See text and ESI,† for further details. (d) Comparison of the diffusion coefficients obtained from long-range sensitive methods (PFG NMR, impedance spectroscopy) and short-range sensitive methods (NMR relaxometry) for LSnPS and LSiPS. The data derived from NMR relaxometry stem from [a] motional narrowing of the 7Li central transition (see ESI,† S4), [b] motional averaging of the quadrupolar interaction, (see ESI,† S4), and [c] longitudinal relaxation (see ESI,† S4). The Arrhenius line obtained for LGPS6 is included as well for comparison. | ||
Impedance spectroscopy was applied in order to study the ionic conductivity of Li10SnP2S12 for which dense ceramic samples could be obtained. Fig. 5a shows the temperature-dependent total conductivity and the bulk conductivity extracted from the impedance spectroscopy measurements for Li10SnP2S12 (see ESI,† for details). The bulk conductivity is activated with 0.25 eV, the room-temperature conductivity amounts to 4 mS cm−1, again in remarkably good agreement with the values predicted from MD simulations3 (0.24 eV and 6 mS cm−1). For comparison, the bulk conductivity reported by Bron et al. for LSnPS was 7 mS cm−1.4 Table S2 (see ESI,† S3) summarizes the bulk conductivity data for LGPS-type electrolytes as obtained in our measurements. In order to determine the electronic contribution to the total conductivity, a dc polarization measurement was carried out at 473 K by applying a small current of 1 nA to a symmetric cell with ion blocking Au electrodes Au|LSnPS|Au. Fig. 5b shows the polarization curve. For a good solid electrolyte with vanishingly small electronic contribution one not only expects a considerable polarization but also a small chemical diffusion coefficient. Accordingly, a steady state could not be attained within reasonable waiting time, but an upper limit of the electronic conductivity corresponding to an electronic transference number of tEON < 1 PPM could be safely determined from the absolute voltage values as well as from the time behaviour13 (linear fit in Fig. 5c, cf. ESI,† S5). Thus, LSnPS can be considered as a purely ionic conductor. The impedance of the pellet prior to and after the dc polarization measurement was equal within 1%. For LSiPS, owing to the instability at sintering temperatures, the preparation of phase pure dense ceramic samples suitable for precise impedance spectroscopic experiments failed and we hence concentrate on the NMR results.
Fig. 5d summarizes the results obtained from PFG NMR, NMR relaxometry, and conductivity measurements for LSiPS and LSnPS. For comparison, the jump rates τ−1 and the conductivities σdc were appropriately transformed into diffusion coefficients Duc (uncorrelated diffusion coefficient) and Dσ (conductivity diffusion coefficient) using the Einstein–Smoluchowski relation Duc = a2/6 × τ−1(jump distance a) and the Nernst–Einstein relation Dσ = kBT/(Nq2) × σdc (number density of Li+N, charge of Li+q, Boltzmann's constant kB). For the calculation, N and an average jump distance of a ∼ 2Å were deduced from the structure. Obviously, for both Li10SnP2S12 and Li11Si2PS12 the macroscopically observed tracer diffusion can be traced back to 3D Li hopping in the bulk lattice with activation energies of 0.25(1) eV and 0.20(1) eV, respectively. The correlation factor f and the Haven ratio HR connecting Duc and Dσ with Dtrvia Dtr = f × Duc = HR × Dσ are on the order of unity as expected for simple diffusion mechanisms. In view of the very good agreement between the results obtained from NMR techniques and impedance spectroscopy in the case of LGPS and LSnPS, we have calculated the expected bulk conductivity of LSiPS from the Arrhenius line obtained from NMR data (Fig. 5d) using the Nernst–Einstein relation and included it in Fig. 5a. For comparison with other non-LGPS-type electrolytes, the conductivity of the best oxidic solid electrolyte reported to date, Li7La3Zr2O12, is included as well.14 For further comparison, Li7La3Zr2O12 shows a conductivity which is almost identical with that of the well-known superionic conductor Li3N, but significantly lower than that of the LGPS-type electrolytes reported in this study.15
Conclusions
The transport properties of Li11Si2PS12, the new tetragonal Si-analogue of the ultrafast electrolyte Li10GeP2S12 are reported, which was obtained under high-pressure conditions for the first time and shown to exhibit the highest room temperature conductivity of any Li solid electrolyte known to date. The structure and Li ion dynamics were characterized in comparison with the Ge-containing parent compound and the Sn analogue, which was comprehensively characterized as well. The Li ion dynamics were elucidated by both long-range sensitive and short-range sensitive techniques and the long-range transport was traced back to Li hopping in the crystalline lattice. In agreement with theoretical predictions, the diffusivity of the Si-compound exceeds that of the Ge-compound while the Sn-analogue shows a lower diffusivity. A clear correlation between the unit cell volume and the diffusivity was observed. Studies of how far the LMePS family is applicable for specific problems of energy research is primarily a question of mechanical stability (e.g. sinterability) and chemical stability of specific contacts. Such studies are currently underway, but out of scope of this report. In summary, the observed high ionic conductivity and earth-abundance of its constituents renders the Ge-free LGPS-type electrolyte Li11Si2PS12 a promising candidate for the development of a new generation of all-solid-state batteries.Acknowledgements
Financial support was granted by the Max Planck Society, the University of Munich (LMU), the Center for NanoScience (CeNS), and the Deutsche Forschungsgemeinschaft (DFG) through the Cluster of Excellence “Nanosystems Initiative Munich” (NIM). B. V. L. gratefully acknowledges financial support by the Fonds der Chemischen Industrie. We thank K. Schunke and U. Engelhardt for help with the high-pressure experiments.Notes and references
- N. Kayama, K. Homma, Y. Yamakawa, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama, K. Kawamoto and A. Mitsui, Nat. Mater., 2011, 10, 682 CrossRef PubMed.
- Theoretical studies on Li10GeP2S12: (a) S. Adams and R. P. Rao, J. Mater. Chem., 2012, 22, 7687 RSC; (b) Y. Mo, S. P. Ong and G. Ceder, Chem. Mater., 2012, 24, 15 CrossRef CAS.
- S. P. Ong, Y. Mo, W. D. Richards, L. Miara, H. S. Lee and G. Ceder, Energy Environ. Sci., 2013, 6, 148 CAS.
- P. Bron, S. Johansson, K. Zick, J. Schmedt a. d. Günne, S. Dehnen and B. Roling, J. Am. Chem. Soc., 2013, 135, 15694 CrossRef CAS PubMed.
- Preliminary results of us on LSnPS and LSiPS had been reported on the occasion of the 112th Bunsentagung, Karlsruhe, Germany (May 10th 2013), as well as at the 19th International Conference on Solid State Ionics, Kyoto, Japan (June 3rd 2013).
- A. Kuhn, V. Duppel and B. V. Lotsch, Energy Environ. Sci., 2013, 6, 3548 CAS.
- M. Murayama, R. Kanno, M. Irie, S. Ito, T. Hata, N. Sonoyama and Y. Kawamoto, J. Solid State Chem., 2002, 168, 140 CrossRef CAS.
- A. Kuhn, J. Köhler and B. V. Lotsch, Phys. Chem. Chem. Phys., 2013, 15, 11620 RSC.
- R. Kanno, 19th International Conference on Solid State Ionics, Kyoto, 2013 Search PubMed.
- R. Kanno and M. Murayama, J. Electrochem. Soc., 2001, 148, A742 CrossRef CAS PubMed.
- M. D. Lind and S. Geller, J. Chem. Phys., 1969, 51, 348 CrossRef CAS PubMed.
- N. Bloembergen, E. M. Purcell and R. V. Pound, Phys. Rev., 1948, 73, 679 CrossRef CAS.
- J. Maier, in Modern Aspects of Electrochemistry, ed. C. Vayenas, R. E. White and M. E. Gamboa-Aldeco, Springer, 2007, vol. 41, p. 95 Search PubMed.
- R. Murugan, V. Thangadurai and W. Weppner, Angew. Chem., Int. Ed., 2007, 46, 7778 CrossRef CAS PubMed.
- A. Rabenau, Solid State Ionics, 1982, 6, 277 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, Rietveld refinement results, supplementary tables, NMR relaxometry details, impedance spectroscopy details, determination of electronic transference number, SEM-EDX, electrochemical stability. See DOI: 10.1039/c4cp02046d |
| This journal is © the Owner Societies 2014 |
