Pure and Zn-doped Pt clusters go flat and upright on MgO(100)†
Abstract
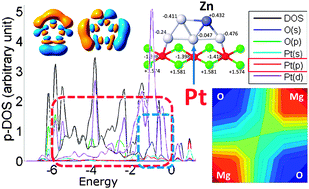
Pure and doped sub-nanoclusters can exhibit superb catalytic activity, which, however, strongly depends on their size, shape, composition, and the nature of the support. This work is about surface-deposited sub-nano Pt-based clusters, which are promising catalysts for the reactions of dehydrogenation. Using density functional theory and ab initio calculations, and an ab initio genetic algorithm for finding the global minima of clusters, we found a peculiar effect that Pt5 and Pt4Zn clusters exhibit upon deposition on MgO(100). Both of them change shapes from the gas phase 3-D form to a planar form, and they stand upright on the support. Several reasons are responsible for this behaviour. In part, clusters go flat due to the electron transfer from the support. Indeed, the anionic Pt5− and Pt4Zn− species are flat also in the gas phase. Charging induces the second-order Jahn–Teller effect (or partial covalency) facilitated by the recruitment of the higher-energy 6p atomic orbitals on Pt into the valence manifold, and that is the reason for the planarization of the anions. Secondly, clusters maximize interactions with the surface O atoms (resulting in further favouring of 2-D structures over 3-D), and avoid contacts with surface Mg atoms (resulting in upright morphologies).
- This article is part of the themed collection: Size Selected Clusters and Particles: From Physical Chemistry to Catalysis

 Please wait while we load your content...
Please wait while we load your content...