Partitioning of prototropic species of an anticancer drug ellipticine in bile salt aggregates of different head groups and hydrophobic skeletons: a photophysical study to probe bile salts as multisite drug carriers†
Abstract
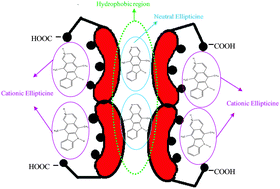
The entrapment of neutral and cationic species of an anticancer drug, namely ellipticine and their dynamic features in different bile salt aggregates have been investigated for the first time using steady state and time-resolved fluorescence spectroscopy. Because ellipticine exists in various prototropic forms under physiological conditions, we performed comparative photophysical and dynamical studies on these prototropic species in different bile salts varying in their head groups and hydrophobic skeletons. We found that the initial interaction between ellipticine and bile salts is governed by the electrostatic forces where cationic ellipticine is anchored to the head groups of bile salts. Bile salts having conjugated head groups are better candidates to bind with the cationic species than those having the non-conjugated ones. The fact implies that binding of cationic species to different bile salts depends on the pKa of the corresponding bile acids. The hydrophobic interaction dominates at higher concentrations of bile salts due to formation of aggregates and results in entrapment of neutral ellipticine molecules according to their hydrophobicity indices. Thus bile salts act as multisite drug carriers. The rotational relaxation parameters of cationic ellipticine were found to be dependent on head groups and the number of hydroxyl groups on the hydrophilic surface of bile salts. Cationic ellipticine exhibits a faster rotational relaxation in the tri-hydroxy bile salt aggregates than in di-hydroxy bile salts. We interpreted this observation from the fact that tri-hydroxy bile salts hold a higher number of water molecules in their hydrophilic surface offering a less viscous environment for ellipticine compared to di-hydroxy bile salts. Surprisingly, the neutral ellipticine molecules display almost the same rotational relaxation in all the bile salts. The observation indicates that after intercalation inside the hydrophobic pocket, neutral ellipticine molecules experience similar confinement in all the bile salts.

 Please wait while we load your content...
Please wait while we load your content...