Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Molecular motors pulling cargos in the viscoelastic cytosol: how power strokes beat subdiffusion
Igor
Goychuk
*a,
Vasyl O.
Kharchenko
b and
Ralf
Metzler
ac
aInstitute for Physics and Astronomy, University of Potsdam, Karl-Liebknecht-Str. 24/25, 14476 Potsdam-Golm, Germany. E-mail: igoychuk@uni-potsdam.de; Fax: +49-331-977-1045; Tel: +49-331-977-5614
bInstitute of Applied Physics, Natl. Acad. Sci. Ukraine, Sumy, Ukraine
cDepartment of Physics, Tampere University of Technology, Korkeakoulunkatu 3, 33101 Tampere, Finland
First published on 26th June 2014
Abstract
The discovery of anomalous diffusion of larger biopolymers and submicron tracers such as endogenous granules, organelles, or virus capsids in living cells, attributed to the viscoelastic nature of the cytoplasm, provokes the question whether this complex environment equally impacts the active intracellular transport of submicron cargos by molecular motors such as kinesins: does the passive anomalous diffusion of free cargo always imply its anomalously slow active transport by motors, the mean transport distance along microtubule growing sublinearly rather than linearly in time? Here we analyze this question within the widely used two-state Brownian ratchet model of kinesin motors based on the continuous-state diffusion along microtubules driven by a flashing binding potential, where the cargo particle is elastically attached to the motor. Depending on the cargo size, the loading force, the amplitude of the binding potential, the turnover frequency of the molecular motor enzyme, and the linker stiffness we demonstrate that the motor transport may turn out either normal or anomalous, as indeed measured experimentally. We show how a highly efficient normal active transport mediated by motors may emerge despite the passive anomalous diffusion of the cargo, and study the intricate effects of the elastic linker. Under different, well specified conditions the microtubule-based motor transport becomes anomalously slow and thus significantly less efficient.
1 Introduction
The thermally driven Brownian motion represents a ubiquitous transport mechanism in aqueous solution, especially for small particles such as metal and salt ions, aminoacids, sugar molecules, and even for larger particles such as transcription factors, enzymes, lipid granules, or RNAs (on scales larger than their gyration radius). The mean squared displacement (MSD) covered by a Brownian particle without external bias (〈r〉 = 0) scales linearly with time, 〈(δr)2〉 = 〈r2〉 − 〈r〉2 ∝ Dt. If an external force fext acts on the particle, the mean distance in the direction of the bias also scales linearly with time, 〈r〉 ∝ fextt/η. The diffusion coefficient D, the viscous friction coefficient η, and the temperature T are then related by the Einstein–Stokes relation D = kBT/η, where kB is the Boltzmann constant. This relation manifests the classical fluctuation–dissipation theorem (FDT) at local thermal equilibrium.1 The Stokes formula η = 6πζa relates the viscous friction with the viscosity ζ of the medium and the radius a for spherical particles in simple fluids. The mobility μ = 1/η and diffusion coefficient of a particle are thus inversely proportional to its size.Molecular transport inside living biological cells comprises both passive and active motion.2 In complex molecularly crowded polymeric fluids such as the cellular cytosol,3 the above simple linear dependence of the friction coefficient on the particle size generally breaks down, and the effective friction coefficient ηeff may even be exponentially enhanced in the liquid, with respect to the one in water by many orders of magnitude. This effect depends on the particle size, the correlation length of the polymeric fluid, and other parameters.4,5 For instance, if for a calcium ion the viscosity in the cytosol of a cell is essentially the same as in water, a vesicle or magnetosome with radius a > 100 nm may rather experience an effective viscosity comparable to the one of glycerol which is some 1500× more viscous than the one of water at room temperature, or even honey (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× more viscous), as extrapolated from recent systematic studies in ref. 6 for a more simple system. Even for particles of a typical size of a globular protein (a ∼ 2.5 nm) the enhancement factor can be as large as 750, as derived in ref. 7 from the experimental data in ref. 8.
000× more viscous), as extrapolated from recent systematic studies in ref. 6 for a more simple system. Even for particles of a typical size of a globular protein (a ∼ 2.5 nm) the enhancement factor can be as large as 750, as derived in ref. 7 from the experimental data in ref. 8.
Indeed, the passive diffusion of submicron particles in living cells slows down tremendously, if to consider it as a normal diffusion process on the macroscale, with respect to the one in pure water. However, a more dramatic effect is that the cytosol actually behaves as a viscoelastic polymeric fluid and displays profound memory effects. Viscoelasticity can cause anomalous diffusion in the subdiffusive range, displayed by the sublinear scaling of the MSD, 〈(δr)2〉 ∝ Dαtα with 0 < α < 1, where the anomalous diffusion coefficient is Dα. Such subdiffusion was established in numerous experiments based on a large range of tracer particles both in the cytosol of living cells and in controlled crowded environments in vitro.8–26 Although subdiffusion mechanisms other than viscoelasticity also appear relevant to explain experimental results, such as continuous time random walk-type motion,19,21–23 and these different types of anomalous diffusion may even mix in some systems,19,22 on time scales relevant to our study the viscoelastic nature of the subdiffusion appears to be well established both in vivo and in control experiments in vitro.16,19–23,25–27
Viscoelastic subdiffusion is related to the famed fractional Brownian motion,28,29 as discussed in ref. 30. This connection can be derived31,32 from a Generalized Langevin Equation (GLE)1,33 with a power-law memory kernel and fractional Gaussian thermal noise,30,34 see below. Dynamically the GLE governed motion with power-law correlations represents a well-founded approach with deep roots in statistical mechanics.33,35,36 As shown in the above experiments, the highly crowded cytosol37,38 is such a viscoelastic environment, and we are thus going to use the GLE approach with power-law correlations in the present work. As a guiding example, we consider the subdiffusive motion of magnetosomes with submicron radius a = 300 nm and chains thereof consisting of up to 8 magnetosomes investigated experimentally in ref. 17. These magnetosomes perform passive subdiffusion with anomalous diffusion exponent α ≈ 0.4 in the intact cytosol. Their motion is characterized by subdiffusion coefficients as small as D0.4 ∼ 10−16 m2 s−0.4 = 100 nm2 s−0.4 and even smaller, depending on the number of magnetosomes in the chain. Therefore, the corresponding subdiffusion spreading within one second is of the order of merely 10 nm.
Without help of active transport by molecular motors such particles would be practically localized on even appreciably long time scales. Pulling by molecular motors under consumption of energy provided by ATP is thus crucial for the delivery of such and similar cargos to more distant locations in living cells.39,40 Given the coupling of such cargos to the viscoelastic properties of their environment in the cytosol and the resulting passive subdiffusion of cargos, the immediate question arises: how does this affect the active transport by molecular motors? This is the focus of the present work.
The theory of molecular motors viewed as Brownian stochastic engines is well developed for Markovian dynamics in absence of any memory effects.41–48 Generalizing these models systematically to the motor motion in viscoelastic media was started to be studied in ref. 34 and 49–53 by extending several popular models of Brownian ratchets48 towards the dynamics with long-range viscoelastic memory. Such ratchets are indeed closely related to molecular motors.41–45,48,54 Within the framework of such ratchet models one can think of the motion of a compound, tightly coupled motor–cargo particle as one moving in a periodic external force field provided by interaction with the polymeric fibers of the cytoskeleton, e.g., the microtubules in the case of kinesin motors considered here. A generalization of such a standard continuous diffusion ratchet model of molecular motors to include viscoelastic subdiffusion has been put forward recently.55 This model explains a number of experimental facts, in particular, that the transport by molecular motors in the viscoelastic cytosol may be both normal and anomalously slow, depending on physical parameters such as the motor's turnover frequency and the cargo size.
In the present work, to become more realistic we establish a further generalization by considering the motor-mediated transport of large subdiffusive cargos, that are attached to the motor by elastic linkers. That is, we consider motor and cargo as two independent particles and thus relax the assumption of the absolutely rigidly linked motor–cargo compound. Similar elastic linker models were considered for the normal diffusion of both cargo and motor in dilute solvent conditions.56 In our approach we assume that the passive diffusion of the motor is normal, without memory effects, when it is not attached to a cargo particle. We characterize the fact that the motor moves in the viscoelastic cytosol and not just water by a significantly reduced (by a factor of 10) diffusion coefficient with respect to the one expected in water. This assumption is justified by the relatively small size of the motor protein itself. However, the coupling to the subdiffusive, comparatively large cargo enforces the subdiffusion of the coupled motor–cargo unit, as long as it is not coupled to a microtubule. Considering experimentally relevant elastic constants of the motor–cargo linker from ref. 18, 57 and other realistic motor parameters we confirm the coexistence of normal and anomalous motor transport for certain parameter values similar to the findings of ref. 55. However, as we discuss here, in this more general setup of an elastic coupling between motor and cargo, the anomalous transport regime becomes in fact reinforced. It is shown to emerge already for motor turnover frequencies of the order of 100 Hz for sufficiently large cargos of a typical radius a = 300 nm. However, even for such large cargo particles the transport can become normal if the turnover frequency is lowered to 10 Hz. Generally, the dependence of the effective subdiffusive transport exponent on the cargo size and the motor turnover frequency can provide a decisive experimental support for anomalous motor transport in living cells.
2 Theory and modeling
Diffusion in complex viscoelastic fluids such as the cytosol is commonly described by the GLE.58,59 We here consider overdamped dynamics of particles, neglecting inertial effects, as appropriate for the low Reynolds number limit for submicron particles in the cytosol. We use the single Cartesian coordinate y for such a particle. It corresponds to the chemical coordinate of the motion along the microtubule when the particle becomes attached to the motor moving on the microtubule. The GLE then reads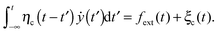 | (1) |
| 〈ξc(t)ξc(t′)〉 = kBTηc(|t − t′|), | (2) |
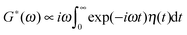 , from measured particle trajectories.
, from measured particle trajectories.
The frequency dependence of the complex shear modulus is commonly used to characterize viscoelastic materials.8,11,12,58–60 In particular, the frequently observed power-law scaling G*(ω) ∝ (iω)α with 0 < α < 1 corresponds to subdiffusion of a free particle in this medium, 〈(δx)2〉 ∝ Dαtα, with fractional diffusion coefficient Dα and power-law memory kernel, η(t) ∝ ηα/tα, where ηα is the fractional friction coefficient obeying the fractional Einstein relation Dα = kBT/ηα.34 In the macroscopic theory of viscoelasticity, similar memory kernels were introduced by A. Gemant61 as a generalization of the simplest Maxwell model with exponential memory decay.62 In practice, the power-law scaling extends over a finite number of time and frequency decades. A high-frequency (short-memory) cutoff reflects the molecular nature of the condensed medium. A low-frequency, or long-memory cutoff guarantees that the macroscopic friction coefficient 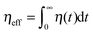 remains finite, reflecting the finite viscosity of any fluid on the macroscale.30,34 The intermediate power-law scaling gives rise to subdiffusion on a transient time scale of up to several minutes, depending on the particle size, and this can establish subdiffusion as a primary passive transport mechanism for submicron particles on the mesoscale inside biological cells.
remains finite, reflecting the finite viscosity of any fluid on the macroscale.30,34 The intermediate power-law scaling gives rise to subdiffusion on a transient time scale of up to several minutes, depending on the particle size, and this can establish subdiffusion as a primary passive transport mechanism for submicron particles on the mesoscale inside biological cells.
The mathematical model of a strictly algebraically decaying memory kernel corresponds to the fractional Gaussian noise (fGn) model of thermal noise. fGn presents a time-derivative of the fractional Brownian motion (fBm). It was introduced by Mandelbrot and van Ness29 who popularized the concept mathematically introduced by Kolmogorov.28 Both can be characterized by the Hurst exponent H′ = 1 − α/2, where α is the anomalous diffusion exponent defined earlier for the MSD. fGn is termed persistent for 1/2 < H′ < 1, i.e., it exhibits positive correlations. It corresponds to the sub-Ohmic model of thermal baths consisting of harmonic oscillators.63 The corresponding GLE can be derived from a purely dynamic hyper-dimensional Hamiltonian model assuming merely initial canonical distribution of thermal bath oscillators at a given temperature, like in a typical molecular dynamics setup.33,63 The solution of GLE (1) is then also fBm, but anti-persistent and subdiffusive, with Hurst exponent H = α/2. This transformation occurs due to the friction with algebraically decaying memory:32 high noise values correspond to a high friction. Importantly, this friction memory is at the heart of the very phenomenon of viscoelasticity. Experimental values of α vary significantly and are in the range α = 0.2–1.23 For example, for the intact cytoskeleton in ref. 17, α = 0.4. About the same value can be derived from experimental data in ref. 18, namely from the power spectrum S(ω) of the transversal position fluctuations of the melanosome particles (size a = 250 nm), that are elastically attached to the motor proteins walking along the microtubules. For sufficiently large frequencies (exceeding the inverse relaxation time scale in a parabolic potential well), S(ω) ∝ 1/ωb, with b = 1 + α, and the experiment yields b = 1.41 ± 0.02. We use α = 0.4 as an experimentally relevant numerical value in what follows.
2.1 Earlier modeling
The simplest way to model the action of molecular motors driving a cargo within the GLE approach is to approximate their collective action in terms of a time-dependent random force fext(t), which itself exhibits a long range memory and is power-law correlated, 〈fext(t′)fext(t)〉 ∝ 1/|t − t′|γ, with 0 < γ < 1. This can lead to superdiffusion,64 〈δy2〉 ∝ tβ, with β = 2α − γ > 1,31,64 for α > 0.5. Notice, however, that β in this approach can only take the maximal value 2α for γ → 0. This corresponds to a strict 1/f noise property of the driving force fext(t) generated by motors with almost non-decaying correlations. The origin of this limit can be easily understood when we consider the transport by a time-alternating force ±f0 in opposite directions. Then, 〈δy(t)〉 ∝ ±f0tα, for a given force realization, and after averaging over the driving force fluctuations 〈〈δy(t)〉2〉f0 ∝ t2α. Any decaying correlations of the alternating driving force render the effective diffusion exponent smaller than βmax = 2α. Clearly, within such a description superdiffusion caused by the activity of molecular motors could only be possible for α > 0.5, at odds with experimental results showing β ∼ 1.2–1.4 for α = 0.4.17 Similarly, the experimental results of ref. 65 cannot be explained within such a model, either: as shown in Fig. 3 in that reference, the passive subdiffusion of lipid droplets with α ≈ 0.58 is changed to superdiffusion with β ≈ 1.74 when they are actively transported by molecular motors. Compared with experimental evidence, the approach in terms of a time-dependent random force is therefore too simple although it provides a number of useful insights.2.2 Our model
We here consider a generalization of the viscoelastic motor model presented in ref. 55. To start with, we assume that a motor with coordinate x along a microtubule elastically couples to the cargo and exerts the force fext = kL(x − y) on it, where the spring constant is kL. The motor itself undergoes diffusion, most generally characterized by a friction memory η(t), on the microtubule. The motor-microtubule binding potential U(x,ζ(t)) reflects the spatial periodicity L of the microtubule. The potential depends on the dynamic motor conformation ζ(t), the motor's internal degree of freedom. Our model for the coupled equations of motor and cargo coordinates y and x then reads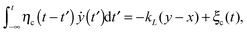 | (3a) |
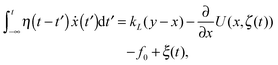 | (3b) |
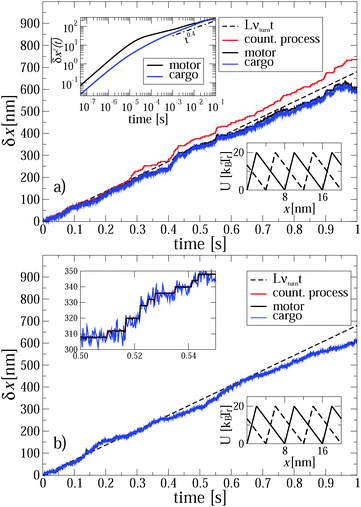 | ||
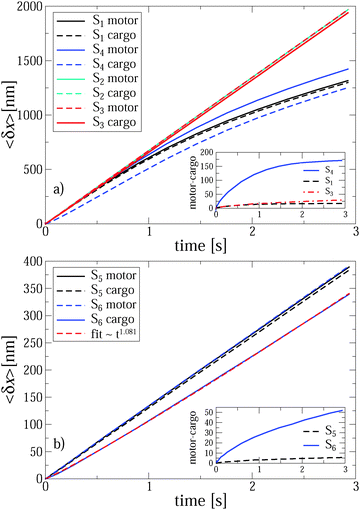
| Fig. 1 Positions of the motor (black line) and cargo (blue line) versus time for a single trajectory realization in the case of anomalous transport (part a, set S1, see Table 1 for the parameters corresponding to various sets Si) and normal transport (part b, set S2. Turnover frequency νturn = 85 Hz). The realizations of counting process (number of potential switches multiplied with potential half-period L/2) are depicted by red lines (difficult to detect in part b because of a perfect synchronization). The broken black lines depict the dependence of the averaged (over many trajectory realizations) position of motor on time in the case of a perfect synchronization (ideal power stroke like mechanism). The upper inset in part a shows diffusion of coupled motor and cargo in the absence of binding potential. The position variances were obtained using a corresponding single-trajectory time averaging, as described in the text. The upper inset in the part b magnifies a part of motor and cargo trajectories making the step-wise motion of motor obvious. It is perfectly synchronized with the potential switches. Cargo randomly fluctuates around the motor position. The lower insets show two conformations of the binding potential. | ||
To be more specific, we consider the piecewise linear sawtooth potential with amplitude U0 and period L. The minimum divides the period in the ratio p![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and we take the particular value p = 3. This asymmetry defines the natural direction of transport in direction of positive x. The maximal possible load, or stalling force for this potential at zero temperature (i.e., without presence of thermal noise) is easy to deduce, namely, fstall0(T = 0) = (p + 1)U0/(pL). In units of thermal energy at room temperature, kBTr = 4.1 × 10−21J = 4.1 pN nm, L = 8 nm, we find fstall0(T = 0) ≈ 0.6833U0/(kBTr) pN for p = 3. For T = Tr the value for fstall0 will be lower, see below. We choose U = 20kBTr in our simulations. This corresponds to fstall0(T = 0) = 13.67 pN, about twice the maximal loading force of kinesin II at physiological temperatures. This choice leads to a reasonable stalling force at room temperature.
1, and we take the particular value p = 3. This asymmetry defines the natural direction of transport in direction of positive x. The maximal possible load, or stalling force for this potential at zero temperature (i.e., without presence of thermal noise) is easy to deduce, namely, fstall0(T = 0) = (p + 1)U0/(pL). In units of thermal energy at room temperature, kBTr = 4.1 × 10−21J = 4.1 pN nm, L = 8 nm, we find fstall0(T = 0) ≈ 0.6833U0/(kBTr) pN for p = 3. For T = Tr the value for fstall0 will be lower, see below. We choose U = 20kBTr in our simulations. This corresponds to fstall0(T = 0) = 13.67 pN, about twice the maximal loading force of kinesin II at physiological temperatures. This choice leads to a reasonable stalling force at room temperature.
In this work, we neglect memory effects for the motor particle due to its relatively small size and choose the δ-form η(t) = 2ηmδ(t) with regular Stokes friction ηm = 6πamζw, where am is the effective radius of the motor molecule and ζw = 1 mPa s. We take am = 100 nm, about 10 times larger than the linear geometrical size of the kinesin molecule in order to account for the enhanced effective viscosity experienced by the motor in the cytosol compared to its value in water. Furthermore, we use the characteristic time scale τm = L2ηm/U0* to scale time in the numerical simulations, where U0* = 10kBTr. For the above parameters, τm ≈ 2.94 μs. Distance is scaled in units of L, and elastic coupling constants in units of U0*/L2 ≈ 0.64 pN nm−1. Moreover, we single out the purely viscous component in the memory kernel of cargo as follows: ηc(t) = 2ηcδ(t) + ηmem(t), where ηmem(t) = ηα/(Γ(1 − α)tα).
It should be stressed that even for this particular choice of the memory kernels the studied model cannot be compared directly with the model in ref. 55. Indeed, if we wanted to exclude the dynamics of the cargo variable by projecting the whole dynamics onto the subspace of the motor particle alone, such a reduced dynamics of the motor particle would be described by the Laplace transformed, reduced memory kernel ![[small eta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e110.gif) red(s) = ηm + kL/[s + kL/(ηc +
red(s) = ηm + kL/[s + kL/(ηc + ![[small eta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e110.gif) mem(s))], where
mem(s))], where ![[small eta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e110.gif) mem(s) = ηαsα−1 is the Laplace transform of the kernel ηmem(t) above. This can be shown for any binding potential U(x,ζ(t)) by formally solving eqn (3a) for the Laplace-transformed variable y(t) using the Laplace-transform method and substituting the result into the Laplace-transformed eqn (3b). Hence, the model of ref. 55 can only be reproduced in the formal limit of an infinitely rigid spring constant with kL → ∞, upon identifying ηm + ηc with η0 in ref. 55, and ηmem(t) with ηm(t) therein, as well as by adopting the same model of binding potential U(x,ζ(t)). However, we are interested in realistically soft linkers and possible effects they can introduce, especially when a fluctuating binding potential acts on the motor. Indeed, when a normally diffusing particle becomes coupled to an anomalously slow one, who will win and enforce its type of behavior, the normal one, or the anomalous one? Or maybe they will find a compromise? The answer to this question is not trivial. In the absence of the binding potential, one intuitively expects that the anomalously slow diffusing particle will asymptotically enslave the normal particle. However, it is not clear what happens for a fluctuating binding potential. This circle of questions has not been studied before, neither in the theory of anomalous diffusion and transport, nor in the field of molecular motors. To elucidate these points is one of the primary goals in this work. Moreover, in this paper we study a different model of the binding potential, and consider a smaller value of α = 0.4 in numerical simulations.
mem(s) = ηαsα−1 is the Laplace transform of the kernel ηmem(t) above. This can be shown for any binding potential U(x,ζ(t)) by formally solving eqn (3a) for the Laplace-transformed variable y(t) using the Laplace-transform method and substituting the result into the Laplace-transformed eqn (3b). Hence, the model of ref. 55 can only be reproduced in the formal limit of an infinitely rigid spring constant with kL → ∞, upon identifying ηm + ηc with η0 in ref. 55, and ηmem(t) with ηm(t) therein, as well as by adopting the same model of binding potential U(x,ζ(t)). However, we are interested in realistically soft linkers and possible effects they can introduce, especially when a fluctuating binding potential acts on the motor. Indeed, when a normally diffusing particle becomes coupled to an anomalously slow one, who will win and enforce its type of behavior, the normal one, or the anomalous one? Or maybe they will find a compromise? The answer to this question is not trivial. In the absence of the binding potential, one intuitively expects that the anomalously slow diffusing particle will asymptotically enslave the normal particle. However, it is not clear what happens for a fluctuating binding potential. This circle of questions has not been studied before, neither in the theory of anomalous diffusion and transport, nor in the field of molecular motors. To elucidate these points is one of the primary goals in this work. Moreover, in this paper we study a different model of the binding potential, and consider a smaller value of α = 0.4 in numerical simulations.
When the cargo particle is not coupled to the motor (kL → 0), its free diffusion is described by the MSD53
| 〈δy2(t)〉 = 2DctE1−α,2(−[t/τin]1−α), | (4) |
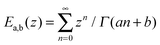 is the generalized Mittag-Leffler function, Dc = kBT/ηc represents the normal diffusion coefficient, and τin = (ηc/ηα)1/(1−α) is a time constant separating initially normal diffusion, 〈δy2(t)〉 ≈ 2Dct at t ≪ τin, and subdiffusion, 〈δy2(t)〉 ≈ 2Dαtα/Γ(1 + α) for t ≫ τin. Furthermore, in accordance with the methodology in ref. 30 and 34 we approximate the memory kernel by a sum of N weighted exponentials
is the generalized Mittag-Leffler function, Dc = kBT/ηc represents the normal diffusion coefficient, and τin = (ηc/ηα)1/(1−α) is a time constant separating initially normal diffusion, 〈δy2(t)〉 ≈ 2Dct at t ≪ τin, and subdiffusion, 〈δy2(t)〉 ≈ 2Dαtα/Γ(1 + α) for t ≫ τin. Furthermore, in accordance with the methodology in ref. 30 and 34 we approximate the memory kernel by a sum of N weighted exponentials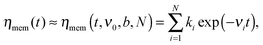 | (5) |
This Markovian embedding approach provides a foundation for excellent numerical treatment of the GLE dynamics with power-law memory kernel. A convenient parameterization uses
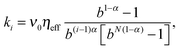 | (6) |
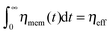 characterizes a largely enhanced macroscopic friction coefficient in the long time limit t ≫ τmax. The number N of auxiliary modes controls the maximal range of the subdiffusive dynamics, which eventually turns normal, 〈δy2(t)〉 ∼ 2Defft for t ≫ τmax with Deff = kBT/(ηeff + ηc). Note that the fractional friction coefficient is related to the effective friction by ηα = ηeffτα−1max/r. Here,
characterizes a largely enhanced macroscopic friction coefficient in the long time limit t ≫ τmax. The number N of auxiliary modes controls the maximal range of the subdiffusive dynamics, which eventually turns normal, 〈δy2(t)〉 ∼ 2Defft for t ≫ τmax with Deff = kBT/(ηeff + ηc). Note that the fractional friction coefficient is related to the effective friction by ηα = ηeffτα−1max/r. Here, 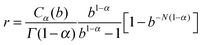 is a numerical coefficient of order of unity, r ≈ 0.93 for N ≥ 5 at α = 0.4 and b = 10, with C0.4(b) ≈ 1.04. The effective relative friction coefficient
is a numerical coefficient of order of unity, r ≈ 0.93 for N ≥ 5 at α = 0.4 and b = 10, with C0.4(b) ≈ 1.04. The effective relative friction coefficient ![[small eta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e110.gif) eff = ηeff/ηc, which is also the effective viscosity of cytosol
eff = ηeff/ηc, which is also the effective viscosity of cytosol ![[small zeta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e126.gif) eff relative to water since we assume that the friction coefficient is proportional to the viscosity, is used as an important parameter in our simulations, see below. It defines the time range of subdiffusion, from τin = τmax/
eff relative to water since we assume that the friction coefficient is proportional to the viscosity, is used as an important parameter in our simulations, see below. It defines the time range of subdiffusion, from τin = τmax/![[small eta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e110.gif) 1/(1−α)eff to τmax. For example, for
1/(1−α)eff to τmax. For example, for ![[small eta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e110.gif) eff = 103 and α = 0.4 one expects that subdiffusion will extend over 5 decades in time. According to this important, nontrivial but simple relation, the relative increase of the effective friction controls the relative time range of subdiffusion,
eff = 103 and α = 0.4 one expects that subdiffusion will extend over 5 decades in time. According to this important, nontrivial but simple relation, the relative increase of the effective friction controls the relative time range of subdiffusion, ![[small eta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e110.gif) 1/(1−α)eff, independently of b and N.
1/(1−α)eff, independently of b and N.
The above approximation allows one to replace the non-Markovian GLE dynamics with a higher dimensional Markovian dynamics by introduction of the N auxiliary Brownian quasi-particles with coordinates yi accounting for internal viscoelastic degrees of freedom.34 In the present case,
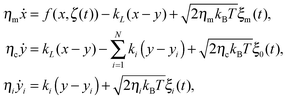 | (7) |
2.3 Thermodynamic efficiency and energetic efficiency of the cargo delivery
The thermodynamic efficiency of anomalous Brownian motors has been addressed quite recently.52,53 It turns out that the general approach developed for normal motors42,67 can be almost directly applied. It yields, however, several important new results in the anomalous transport regime. Generically, the work performed by the potential fluctuations induced by the enzyme turnover, or the input energy pumped into directed motion is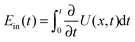 .67 This can be evaluated within the considered model, as a sum of potential energy jumps ΔU(x(ti)) occurring at random instants of time ti marking the cyclic conformational transitions 1 → 2 → 1 → ⋯. This input energy is invested in work against the external loading force f0. The corresponding useful work is Wuse(t) = f0δx(t). The rest is used to overcome the dissipative influence of the environment, and thus converted to heat. The thermodynamic efficiency of isothermal motors is thus Rth(t) = 〈Wuse(t)〉/〈Ein(t)〉, after averaging over many ensemble realizations. Clearly, Rth = 0 for f0 = 0. This is also very clear from an energetic point of view: by relocation from one place to another neither the potential energy of the cargo, nor that of the motor has been changed. Such behavior corresponds to the normal modus operandi of motors as kinesin which is very different from other molecular machines such as ion pumps, which are primarily transferring ions against an electrochemical potential gradient, i.e., effecting an increase of the electrochemical potential of the ions. Anomalously slow transport introduces principally new features for f0 > 0. Namely, for anomalous transport the useful work done against f0 scales sublinearly with time, 〈Wuse(t)〉 ∝ f0tαeff, while the input energy scales linearly, 〈Ein(t)〉 ∝ t, the latter being proportional to the mean number of potential fluctuations.
.67 This can be evaluated within the considered model, as a sum of potential energy jumps ΔU(x(ti)) occurring at random instants of time ti marking the cyclic conformational transitions 1 → 2 → 1 → ⋯. This input energy is invested in work against the external loading force f0. The corresponding useful work is Wuse(t) = f0δx(t). The rest is used to overcome the dissipative influence of the environment, and thus converted to heat. The thermodynamic efficiency of isothermal motors is thus Rth(t) = 〈Wuse(t)〉/〈Ein(t)〉, after averaging over many ensemble realizations. Clearly, Rth = 0 for f0 = 0. This is also very clear from an energetic point of view: by relocation from one place to another neither the potential energy of the cargo, nor that of the motor has been changed. Such behavior corresponds to the normal modus operandi of motors as kinesin which is very different from other molecular machines such as ion pumps, which are primarily transferring ions against an electrochemical potential gradient, i.e., effecting an increase of the electrochemical potential of the ions. Anomalously slow transport introduces principally new features for f0 > 0. Namely, for anomalous transport the useful work done against f0 scales sublinearly with time, 〈Wuse(t)〉 ∝ f0tαeff, while the input energy scales linearly, 〈Ein(t)〉 ∝ t, the latter being proportional to the mean number of potential fluctuations.
By the same token as fractional transport cannot be characterized by a constant mean velocity, it also cannot be characterized by any mean power or useful work in a stationary regime. One way around this is to define a fractional power and fractional efficiency.52,53 Or, one interprets physically the non-constant thermodynamic efficiency Rth. In the present case it decays algebraically in time, Rth(t) ∝ 1/t1−αeff and can still be comparatively high even for large times, as shown below. In the anomalous transport regime, asymptotically Rth(t) → 0 independently of f0. Even though the useful work performed against f0 is always finite, a benchmark of any genuine Brownian motor, it becomes a negligible portion of the input energy in the course of time. The input energy is spent mostly to overcome the dissipative influence of the environment, that effects a hugely enhanced effective viscosity. It is dissipated as heat, Q(t) = 〈Ein(t)〉 − 〈Wuse(t)〉.
The primary task of molecular motors such as kinesin consists in the delivery of various cargos to certain destinations, and not in the increase of their own potential energy. For this reason, numerous Stokes efficiencies have been defined in addition to Rth.68–70 Which of them is most appropriate remains somewhat vague, especially with respect to anomalous transport.53 We proposed a different measure to quantify the motor performance at Rth = 0, namely, the energetic delivery performance.55 It reflects the optimization of the mean delivery velocity per energy spent. Quantifying the net input energy by the number of enzyme turnovers, a natural definition is D = d/(t〈Nturn〉), where d is the delivery distance reached during time t, after 〈Nturn〉 cyclic turnovers on average. Clearly, 〈Nturn〉 = νturnt, and for an ideal motor in the tight coupling power stroke regime, whose processive motion is perfectly synchronized with the turnovers of the ‘catalytic wheel’,71d = Lνturnt, and therefore Dideal = L2νturn/d. For any given d, the increase of νturn leads to a linearly increased delivery performance. However, in reality there will always exist deviations from this idealization.
3 Results and discussion
In our simulations we use N = 10, b = 10, and ν0 = 100 for the Markovian embedding, yielding τmax ≈ 29.4 s for τm = 2.94 μs. The numerical solution of the stochastic differential eqn (7) was achieved via the stochastic Heun method72 in CUDA on NVIDIA Kepler graphical processor units. The time step of the integration was δt = 5 × 10−3 in scaled units, and the terminal time was 106 (2.94 s) in most simulations. We considered a cargo with radius a = 300 nm (ηc/ηm = 3) and two different values of![[small eta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e110.gif) eff = 3 × 104, the ‘larger’ effective viscosity, and
eff = 3 × 104, the ‘larger’ effective viscosity, and ![[small eta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e110.gif) eff = 3 × 103, the ‘smaller’ one. This corresponds to two different values of the subdiffusion coefficient D(1)α ≈ 171 nm2 s−0.4, similar to typical values measured for magnetosomes in ref. 17 and the ten times larger D(2)α ≈ 1710 nm2 s−0.4, corresponding to a smaller particle. Moreover, two different values for the elastic spring constant were used, k(1)L = 0.32 pN nm−1 (‘strong’), which corresponds to measurements in vitro,57 and a ten times softer spring k(2)L = 0.032 pN nm−1 (‘weak’), in accordance with recent results in ref. 18 in living cells. Finally, ν(1)turn = 85 Hz and ν(2)turn = 17 Hz denote the ‘fast’ and ‘slow’ turnover frequencies. According to these parameters we consider the six different parameter sets listed in Table 1.
eff = 3 × 103, the ‘smaller’ one. This corresponds to two different values of the subdiffusion coefficient D(1)α ≈ 171 nm2 s−0.4, similar to typical values measured for magnetosomes in ref. 17 and the ten times larger D(2)α ≈ 1710 nm2 s−0.4, corresponding to a smaller particle. Moreover, two different values for the elastic spring constant were used, k(1)L = 0.32 pN nm−1 (‘strong’), which corresponds to measurements in vitro,57 and a ten times softer spring k(2)L = 0.032 pN nm−1 (‘weak’), in accordance with recent results in ref. 18 in living cells. Finally, ν(1)turn = 85 Hz and ν(2)turn = 17 Hz denote the ‘fast’ and ‘slow’ turnover frequencies. According to these parameters we consider the six different parameter sets listed in Table 1.
| Set |
![[small eta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e110.gif) eff
eff
|
k L /pN nm−1 | ν turn/Hz |
|---|---|---|---|
| S1 | 3 × 104 | 0.320 | 85 |
| S2 | 3 × 103 | 0.320 | 85 |
| S3 | 3 × 103 | 0.032 | 85 |
| S4 | 3 × 104 | 0.032 | 85 |
| S5 | 3 × 104 | 0.320 | 17 |
| S6 | 3 × 104 | 0.032 | 17 |
In Fig. 1a we illustrate both the active and passive motion of the cargo and motor particles for single trajectories. Consider first the case that the motor particle is detached from the microtubule. If motor and cargo are unconnected, the motor particle according to our model assumption would diffuse normally at all times, 〈(δr)2〉 ∝ D1t, while the larger cargo particle would exhibit anomalous diffusion, 〈(δr)2〉 ∝ Dαtα, over a large intermediate time range. Initially, both particles diffuse normally as the viscoelastic friction is not relevant at such early times. Interestingly, a similar feature, i.e., initially normal diffusion without any persistence or anti-persistence in the motion was found experimentally for lipid droplets in ref. 65. When both motor and cargo particles are harmonically coupled, the motor particle initially diffuses normally but eventually becomes enslaved by the cargo particle, and both subdiffuse. This is shown in the top inset of Fig. 1a, where we plot the single trajectory MSD in terms of the sliding average
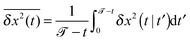 | (8) |
![[scr T, script letter T]](https://www.rsc.org/images/entities/char_e533.gif) this quantity provides the same information as the ensemble average 〈δx2(t)〉, since the considered viscoelastic diffusion is ergodic.30,73,74 We note that, on average, no mean displacement
this quantity provides the same information as the ensemble average 〈δx2(t)〉, since the considered viscoelastic diffusion is ergodic.30,73,74 We note that, on average, no mean displacement 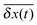 of the motor–cargo complex occurs. This is in accordance with our intuition: the subdiffusing particle eventually enslaves the normal one, in the absence of external forces acting on the latter.
of the motor–cargo complex occurs. This is in accordance with our intuition: the subdiffusing particle eventually enslaves the normal one, in the absence of external forces acting on the latter.
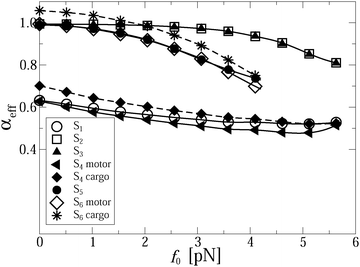
However, once the motor is attached to the microtubular track, it processively steps in the direction defined by the asymmetry of the ratchet potential, and now the cargo has to move along with the motor. This is seen in the main part of Fig. 1a. Initially, the motor steps are perfectly coupled to the potential fluctuations, where it should be noted that a particular realization of the counting process in units of L/2 in this figure is faster than the average value corresponding to the optimal transport distance d = Lν(1)turnt, cf. the broken line. Indeed, different realizations of single enzyme dynamics yield different realizations of the potential fluctuations and the above optimal transport distance is the ensemble average over many trajectories. The particular realization can be both faster and slower, with a typical fluctuation in the number of steps of the order of 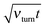 . After some transient time one can clearly see the motor backstepping. Moreover, the cargo particle always fluctuates around the motor position, but on average it lags somewhat behind the motor. It is not obvious from this figure that the transport is anomalously slow and not just corresponds to some suboptimal mean motor velocity v < vopt = Lνturn. However, the transport is indeed anomalous, 〈δx(t)〉 ∝ tαeff, after an initially normal transport regime as can be deduced after averaging over 1000 different trajectory realizations, as shown in Fig. 2. This allows us to extract the anomalous transport exponent αeff. As already verified in our previous studies of viscoelastic subdiffusive dynamics in periodic nonlinear potentials, the value of this αeff is generally time-dependent. However, it relaxes to a long-time limiting value in the course of time, which is displayed in Fig. 3 as function of the load f0 for different sets of parameters.
. After some transient time one can clearly see the motor backstepping. Moreover, the cargo particle always fluctuates around the motor position, but on average it lags somewhat behind the motor. It is not obvious from this figure that the transport is anomalously slow and not just corresponds to some suboptimal mean motor velocity v < vopt = Lνturn. However, the transport is indeed anomalous, 〈δx(t)〉 ∝ tαeff, after an initially normal transport regime as can be deduced after averaging over 1000 different trajectory realizations, as shown in Fig. 2. This allows us to extract the anomalous transport exponent αeff. As already verified in our previous studies of viscoelastic subdiffusive dynamics in periodic nonlinear potentials, the value of this αeff is generally time-dependent. However, it relaxes to a long-time limiting value in the course of time, which is displayed in Fig. 3 as function of the load f0 for different sets of parameters.
It is very important for physical reasons that the cargo lags behind the motor, following at a certain mean distance with fluctuations around it. Therefore, it makes sense to consider two effective exponents αeff for the motor and cargo separately. For a stronger linker, both exponents are very similar because the average distance between the motor and the cargo is relatively small, see the inset in Fig. 2a. Even in the strongly anomalous case S1, the average distance saturates at around δx = 17 nm, which largely exceeds the corresponding amplitude of the thermal fluctuations, given by the root mean square (rms) 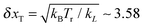 nm. The elastic spring energy corresponding to the above average distance is EL = k(1)L(δx)2/2 ∼ 46.24 pN nm ∼ 11kBTr. It can be easily sustained by a covalent bond. However, for a weaker linker the scenario becomes highly nontrivial and in fact strongly depends on the character of the transport regime. In this case, the difference between two exponents αeff for motor and cargo can be essential, see in Fig. 3 for a larger cargo. For a smaller cargo in the normal transport case S3, the distance is about 29 nm at the end of the simulation, see the inset in Fig. 2a, and the spring energy is about 3.3kBTr only. However, in the strongly anomalous regime S4, the mean distance increases to 170 nm and the spring energy rises accordingly until about 111kBTr. This is already a typical energy of a covalent bond. Therefore, such a linker, considered as globular entropic spring, will be definitely unfolded to reach its maximal length, which can indeed be around 150 nm when it is fully stretched and is sufficiently long.75 However, the most probable scenario is that such a linker will simply break or, alternatively, the cargo will dissociate from the linker and will be lost by the motor. This is the first important result of this work. Namely, to transfer a large cargo in a strongly anomalous transport regime the linker must be sufficiently strong. With these caveats in mind, we consider the case S4 in more detail, as it also reveals another remarkable feature, which is expected to be present for stronger linkers, as well, albeit to a lesser degree. Namely, in the case S4 the motor moves faster than in the case of a stronger linker for S1, see Fig. 2a. This is a remarkable observation, which was not expected a priori. Given this feature, the motor can be expected to perform a larger work against an external load f0 for a weaker linker. Moreover, on decreasing the turnover frequency down to 17 Hz (see the case S6 in Fig. 2b) the motor operates normally, with the same mean velocity as in case S5 corresponding to the optimal motor regime at this frequency. However, the cargo paradoxically enters a super-transport regime with a scaling exponent αeff ≈ 1.081, see also Fig. 3, while moving slower than the motor. This means that the transport exponent β = 2αeff can, in principle, become larger than two, i.e. the observed motor-mediated diffusion of the cargo can be transiently super-ballistic. This is our second nontrivial and surprising result. The explanation of this seemingly paradoxical behavior is quite simple. Namely, it occurs because the distance between the motor and cargo increases sublinearly in time, before entering the saturation regime. To realize this, let us assume first that this mutual distance grows linearly in time. Then, the cargo distance would also grow linearly with an effective velocity equal to the motor velocity minus the velocity of the mutual distance growth. However, if the mutual distance increases only sublinearly, i.e. with a velocity which drops in time, see inset in Fig. 2b, it appears that the cargo moves faster than in the normal transport regime characterized by the effective velocity. Paradoxically, an ever increasing delay of the cargo motion past the optimally operating motor due to the weakness of the motor–cargo linker can be interpreted as a super-transport of cargo. However, upon a saturation of the motor–cargo distance this transient regime will be over. This example shows that it can be very misleading to associate the value of anomalous transport exponent αeff with the speed of transport.
nm. The elastic spring energy corresponding to the above average distance is EL = k(1)L(δx)2/2 ∼ 46.24 pN nm ∼ 11kBTr. It can be easily sustained by a covalent bond. However, for a weaker linker the scenario becomes highly nontrivial and in fact strongly depends on the character of the transport regime. In this case, the difference between two exponents αeff for motor and cargo can be essential, see in Fig. 3 for a larger cargo. For a smaller cargo in the normal transport case S3, the distance is about 29 nm at the end of the simulation, see the inset in Fig. 2a, and the spring energy is about 3.3kBTr only. However, in the strongly anomalous regime S4, the mean distance increases to 170 nm and the spring energy rises accordingly until about 111kBTr. This is already a typical energy of a covalent bond. Therefore, such a linker, considered as globular entropic spring, will be definitely unfolded to reach its maximal length, which can indeed be around 150 nm when it is fully stretched and is sufficiently long.75 However, the most probable scenario is that such a linker will simply break or, alternatively, the cargo will dissociate from the linker and will be lost by the motor. This is the first important result of this work. Namely, to transfer a large cargo in a strongly anomalous transport regime the linker must be sufficiently strong. With these caveats in mind, we consider the case S4 in more detail, as it also reveals another remarkable feature, which is expected to be present for stronger linkers, as well, albeit to a lesser degree. Namely, in the case S4 the motor moves faster than in the case of a stronger linker for S1, see Fig. 2a. This is a remarkable observation, which was not expected a priori. Given this feature, the motor can be expected to perform a larger work against an external load f0 for a weaker linker. Moreover, on decreasing the turnover frequency down to 17 Hz (see the case S6 in Fig. 2b) the motor operates normally, with the same mean velocity as in case S5 corresponding to the optimal motor regime at this frequency. However, the cargo paradoxically enters a super-transport regime with a scaling exponent αeff ≈ 1.081, see also Fig. 3, while moving slower than the motor. This means that the transport exponent β = 2αeff can, in principle, become larger than two, i.e. the observed motor-mediated diffusion of the cargo can be transiently super-ballistic. This is our second nontrivial and surprising result. The explanation of this seemingly paradoxical behavior is quite simple. Namely, it occurs because the distance between the motor and cargo increases sublinearly in time, before entering the saturation regime. To realize this, let us assume first that this mutual distance grows linearly in time. Then, the cargo distance would also grow linearly with an effective velocity equal to the motor velocity minus the velocity of the mutual distance growth. However, if the mutual distance increases only sublinearly, i.e. with a velocity which drops in time, see inset in Fig. 2b, it appears that the cargo moves faster than in the normal transport regime characterized by the effective velocity. Paradoxically, an ever increasing delay of the cargo motion past the optimally operating motor due to the weakness of the motor–cargo linker can be interpreted as a super-transport of cargo. However, upon a saturation of the motor–cargo distance this transient regime will be over. This example shows that it can be very misleading to associate the value of anomalous transport exponent αeff with the speed of transport.
For S1 and f0 = 0 shown in Fig. 1a, αeff ≈ 0.63, both for the motor and cargo. The same value also characterizes the motor in the case S4. However, for the cargo the transport exponent here is larger, αeff ≈ 0.70, even if it moves absolutely slower, an interesting feature by itself. This once again indicates that there is no simple general relation between the value of the transport exponent and the transport speed, contrary to common belief. The observed features explain the origin of the superdiffusive exponent in the range β = 1.2–1.4, in spite of the low value of the free subdiffusion exponent α = 0.4 in ref. 17. However, for a smaller particle (or rather for a smaller ηα in our model) corresponding to the case S2, the transport becomes normal at the same flashing frequency and without biasing back load, f0 = 0, as demonstrated in Fig. 1b. Interestingly, the corresponding single trajectory realization in Fig. 1b does not correspond to a larger transport distance at t = 1 s as compared with the anomalous transport in Fig. 1a. This is simply because the number of turnovers performed until this time is larger in Fig. 1a than in Fig. 1b, for the particular realizations presented. This reflects statistical variations of the corresponding counting process, or, in physical terms, the stochastic nature of single motor proteins. The upper inset in Fig. 1b reveals a perfect synchronization between the potential switches and the motor steps. It renders the red line corresponding to the potential switches barely distinguishable in this plot. One can also see in this upper inset that the cargo particle fluctuates symmetrically around the motor. It does not lag behind the motor on average, in contrast to Fig. 1a. Furthermore, within a transport regime closer to normal transport a much softer linker does not significantly change the results, as demonstrated by Fig. 3 for a smaller cargo. For a larger cargo, though, anomalous transport becomes clearly affected in a very nontrivial way, as discussed above.
Notably, the motor can operate normally if the turnover frequency is reduced, see the results for S5 and S6 at f0 = 0 in Fig. 3. However, the transport of cargo can become both normal (strong linker, S5), or even, seemingly paradoxical, anomalously fast, if to judge from the transport exponent αeff for a weak linker, S6. This super-transport is in fact absolutely slower than the normal one, see Fig. 2b and the related discussion above. These results show that motors can realize both normal and anomalous transport in the viscoelastic cytosol of living cells, where large submicron cargos subdiffuse on the time scale from milliseconds to tens of seconds. Which transport regime will be realized depends specifically on the particle size (or fractional friction coefficient ηα), the enzyme turnover frequency νturn, and on the linker rigidity. This presents another important result of our work.
Furthermore, if a counter-force f0 > 0 is applied, the anomalous transport regime becomes promoted, as shown in Fig. 3. This effect is primarily due to the reduction of the binding potential amplitude U0. Anomalous transport will also emerge immediately for the studied parameters if U0 is decreased, for instance, to the lower value 10kBTr.
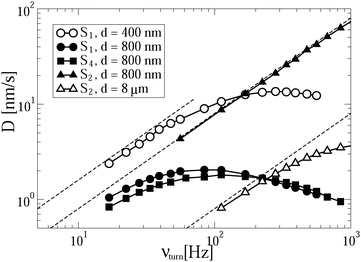
The transport efficiency of cargo delivery in the viscoelastic cytosol can be of the same order as that for the motion in a dilute solution, despite the passive subdiffusion. This efficiency is demonstrated in Fig. 4 for realistic turnover frequencies (lower than 200 Hz). It persists even for large distances of some 8 μm. The discussed power stroke mechanism can thus perfectly overcome the subdiffusive resistance of the medium, if the cargo size is not too large. In the anomalous transport regime with strongly decreased transport efficiency there still exists an optimal turnover frequency, which depends on the delivery distance. Indeed, in the anomalous transport regime with increasing turnover frequency or load even normally operating motors do not have sufficient time to relax down to the potential minimum after each potential jump, or escape events become important. This results in backsteps, and after reaching a maximum versus νturn the delivery efficiency will necessarily drop. In the anomalous transport regime, the optimal νoptturn(d) necessarily decays with increasing delivery distance d. Energetically, it makes then less sense to hydrolyze more ATP molecules for efficient cargo delivery. A corresponding optimization can be important for the cell economy. As expected, anomalous transport on a stronger linker is more efficient for realistic turnover frequencies, a fact that agrees with intuition. This is because the cargo follows at a larger distance from the motor in case of a weaker linker.
The thermodynamic efficiency becomes strongly affected by the fact that an increase of f0 promotes the onset of the anomalous transport regime, as observed in Fig. 3. Even if the transport was normal for f0 = 0 it may become anomalous under a sufficiently strong external load f0. Hence, whenever the transport is anomalous, the thermodynamic efficiency starts to depend on time and vanishes asymptotically. However, it can be quite large even at long times such as tmax ∼ 3 s, see Fig. 5, where it still amounts to some 23% at the maximum, see the results for cases S2 and S3, where the thermodynamic efficiency practically does not depend on the linker rigidity. This is an important result of the present work, which is a priori counterintuitive. Namely, in a highly efficient transport regime, which is closer to normal transport, neither transport exponent αeff (Fig. 3) nor the thermodynamic efficiency are practically affected by the rigidity of the linker. However, in the low efficiency transport regime, the influence of the linker rigidity becomes evident in Fig. 3 and 5. Remarkably, the thermodynamic efficiency of the motor is slightly larger for a weaker linker. This interesting feature is due to the fact that the motor covers larger distances in this case, as shown in Fig. 2. Interestingly, the stalling force does not depend on kL at all for the studied parameters. The dependence of Rth(t,f0) on the load f0 is very illustrative: Rth(t,f0) vanishes not only at f0 = 0, but also at some fstall0(T,U0,νturn). This defines the stalling force, which is time-independent but strongly depends on potential amplitude, temperature, and driving frequency. The thermodynamic efficiency has thus a maximum at some optimal loading force fopt0(t), and Rth(t,fopt0) can be relatively high in the anomalous transport regime, for a sufficiently high potential amplitude U0, comparable with the maximal thermodynamic efficiency of kinesins in the normal regime of about 50%. The dependence of Rth(t,f0) on f0 is strongly asymmetric in the thermodynamically highly efficient regime. However, it becomes more symmetric in a low efficient anomalous regime, where it is described approximately by the parabolic dependence Rth ∝ f0(1 − f0/fstall0)/fstall0 with fopt0 = fstall0/2, and a proportionality coefficient which slowly drops in time. This phenomenon of a time-dependence of Rth(t) can be verbalized as fatigue of molecular motors caused by the viscoelasticity of the cytosol. It can be used as direct means to probe our model experimentally.
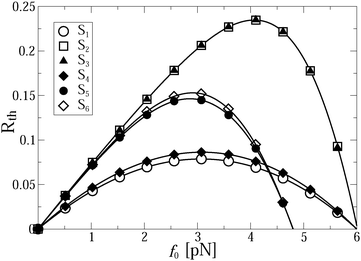 | ||
| Fig. 5 Motor thermodynamic efficiency as function of loading force f0 at the end of simulations (corresponding to tmax = 2.94 s) for different sets. | ||
Indeed, for low-efficient anomalous transport, the dependence of Rth on the load in Fig. 5 is approximately parabolic, with a maximum at fopt0 = fstall0/2. Counterintuitively, it is slightly larger for a softer linker. This is because the useful work was defined as the work performed by motors against f0, and not by the cargo, and that the motors step ahead of their cargos on slightly larger distances for a softer linker. The maximal loading, or stalling force fstall0 is time-independent. It also does not depend on the cargo size. However, it strongly depends on the flashing frequency (Fig. 5), as well as on the potential amplitude and temperature (Fig. 6). Numerical results show that for U0 > Um(νturn)T/Tr,
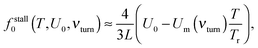 | (9) |
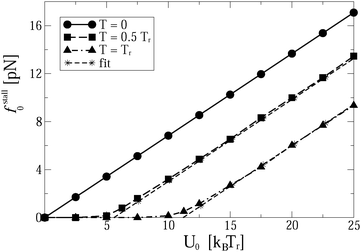 | ||
| Fig. 6 Stalling force as function of barrier height U0 at different temperatures and fixed νturn = 85 Hz. Numerical results are compared with an analytical fit by eqn (9) for U0 > UmT/Tr. | ||
4 Conclusions
In this work, we presented a model for active transport of submicron cargo particles by molecular motors in the viscoelastic cytosol of living cells. This model goes significantly beyond our previous discussion in ref. 55 by the inclusion of a realistic elastic linker between the motor and its cargo, giving rise to a number of surprising effects. When decoupled from the motor, the cargo particles subdiffuse and the motor operates normally. As shown here, the effective transport of the coupled motor–cargo complex may be both normal and anomalous, depending on the physical parameters of the system.The viscoelasticity of the cellular cytosol is taken into account by the formulation of the cargo dynamics in terms of a Generalized Langevin Equation with a memory kernel, which scales in accordance with a power law between two cutoffs. Passive subdiffusion according to our model occurs on time scales between τmin = τmax/ζ1/(1−α)rel and τmax, where ζrel is the effective cytosol viscosity relative to the water viscosity. It is distinctly enhanced, ζrel ≫ 1, for submicron particles. Asymptotically, normal diffusion of the free particle is restored, but with this increased value of ζrel. For α = 0.4 and typical values ζrel ∼ 104 or ζrel ∼ 103, subdiffusion then persists over about 6.5 or 5 decades in time, respectively. Such transient subdiffusion is described by the multidimensional Markovian embedding with a well-controlled accuracy of the approximation. The molecular motor is described by a variant of the standard Markovian model of flashing Brownian motors with a periodic saw-tooth potential, that randomly fluctuates between two realizations differing by phase, such that two potential flashes correspond to one complete enzyme cycle, that can advance the motor by one spatial period. This model describes, in particular, a perfect power stroke ratchet transport in the case of highly processive molecular motors. It explains how a perfect ratchet mechanism can win over subdiffusional restrictions in living cells. A perfect transport regime with maximal transport efficiency emerges when the motion of the motor is locked onto the potential fluctuations effected by the internal motor turnover. As an important new result, we clearly showed that in order to operate efficiently with a non-vanishingly small stalling force in the viscoelastic environment of living cells the amplitude of the binding potential U0 should be no less than around ten kBTr, or 0.25 eV. For a realistic value U0 ∼ 20kBTr ∼ 0.5 eV, with a stalling force about 5–6 pN within the present model, depending on the motor operating frequency it turns out that variation of the spring constant of the linker in the experimentally relevant range kL ∼ 0.03–0.3 pN nm−1 does not change significantly the major results in the efficient transport regime for sufficiently small cargos. However, in a strongly anomalous regime emerging for large cargos the weakness of the linker can distinctly influence the transport. In fact, our results show that a weak linker with kL ∼ 0.03 pN nm−1 might simply not be able to sustain anomalous transport of sufficiently large cargos with a = 300 nm in such a regime. It will be strongly stretched and eventually break, or the cargo will dissociate from it and become lost (other scenarios are also possible). However, if the motor operates slowly and enters the optimal normal regime, such a weak linker may indeed support transport of such large cargos on a smaller spatial scale with cargo entering a paradoxical regime of transient super-transport with αeff larger than one, the origin of which is rationalized within our model. Furthermore, the cargo delivery efficiency is generally larger for a stronger linker, but the thermodynamic efficiency of the motor is somewhat smaller. In our view, these conclusions are interesting and important, and will prompt further studies in this direction.
The crucial point which our model explains is how one and the same motor in the same cell can realize both normal and anomalous transport. The occurrence of a particular transport regime depends on the binding potential amplitude, the fractional frictional strength ηα of the cargo particle (depending on its size), the external loading force f0, and the enzyme turnover frequency. The effective transport exponent αeff can vary from α to one, and this can easily explain the emergence of anomalously fast diffusion with β = 2αeff mediated by motors in the cells with α ≤ 0.5. Strikingly enough, the transport can be perfectly normal (in agreement with many experiments), reflecting an almost perfect synchronization between the enzymatic turnovers and the motor's stepping along microtubule. This can be rationalized within a power-stroke mechanism and explains how a power-stroke like operation of molecular motors can win over passive subdiffusion. Conversely, in the presence of a large cargo or otherwise unfavorable parameters such as a high turnover frequency, the viscoelasticity of the environment may enforce a subdiffusive, anomalous transport by the molecular motor. Then, the average distance traveled by the motor–cargo compound grows slower than linearly in time.
As a final conclusion, we reaffirmed in a more realistic setting the major qualitative results obtained in ref. 55 based on a more simplistic approach. Thus, the occurrence of anomalous versus normal transport depending on the cargo size and the motor turnover frequencies now appears to be well rationalized. In particular, in order to beat subdiffusion with a power stroke one needs an appreciably strong linker between the motor and its cargo.
We hope that these new results will inspire new experiments, as they should be present in more complex models of molecular motors operating in viscoelastic cytosol. We hope that this can be done in a nearest future.
Acknowledgements
Support of this research by the German Research Foundation, Grant GO 2052/1-2, as well as funding from the Academy of Finland (FiDiPro scheme) are gratefully acknowledged.References
- R. Kubo, Rep. Prog. Phys., 1966, 29, 255 CAS.
- P. Nelson, Biological Physics: Energy, Information, Life, Freeman and Co., New York, 2004 Search PubMed.
- K. Luby-Phelps, Mol. Biol. Cell, 2013, 24, 2593 CrossRef CAS PubMed.
- T. Odijk, Biophys. J., 2000, 79, 2314 CrossRef CAS.
- L. Masaro and X. X. Zhu, Prog. Polym. Sci., 1999, 24, 731 CrossRef CAS.
- R. Holyst, et al. , Phys. Chem. Chem. Phys., 2009, 11, 9025 RSC.
- I. Goychuk, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2012, 86, 021113 CrossRef.
- G. Guigas, C. Kalla and M. Weiss, Biophys. J., 2007, 93, 316 CrossRef CAS PubMed.
- F. Amblard, et al. , Phys. Rev. Lett., 1996, 77, 4470 CrossRef CAS.
- M. J. Saxton and K. Jacobson, Annu. Rev. Biophys. Biomol. Struct., 1997, 26, 373 CrossRef CAS PubMed.
- H. Qian, Biophys. J., 2000, 79, 137 CrossRef CAS.
- S. Yamada, D. Wirtz and S. C. Kuo, Biophys. J., 2000, 78, 1736 CrossRef CAS.
- A. Caspi, R. Granek and M. Elbaum, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2002, 66, 011916 CrossRef.
- I. M. Tolić-Nørrelykke, E.-L. Munteanu, G. Thon, L. Oddershede and K. Berg-Sørensen, Phys. Rev. Lett., 2004, 93, 078102 CrossRef.
- I. Golding and E. C. Cox, Phys. Rev. Lett., 2006, 96, 098102 CrossRef PubMed.
- J. Szymanski and M. Weiss, Phys. Rev. Lett., 2009, 103, 038102 CrossRef.
- D. Robert, Th.-H. Nguyen, F. Gallet and C. Wilhelm, PLoS One, 2010, 4, e10046 Search PubMed.
- L. Bruno, M. Salierno, D. E. Wetzler, M. A. Desposito and V. Levi, PLoS One, 2011, 6, e18332 CAS.
- J. H. Jeon, V. Tejedor, S. Burov, E. Barkai, C. Selhuber-Unkel, K. Berg-Sørensen, L. Oddershede and R. Metzler, Phys. Rev. Lett., 2011, 106, 048103 CrossRef.
- J. H. Jeon, M. Leijnse, L. B. Oddershede and R. Metzler, New J. Phys., 2013, 15, 045011 CrossRef.
- E. Barkai, Y. Garini and R. Metzler, Phys. Today, 2012, 65(8), 29 CrossRef CAS PubMed.
- S. M. A. Tabei, S. Burov, H. Y. Kim, A. Kuznetsov, T. Huynh, J. Jureller, L. H. Philipson, A. R. Dinner and N. F. Scherer, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 4911 CrossRef PubMed.
- F. Höfling and T. Franosch, Rep. Prog. Phys., 2013, 76, 046602 CrossRef PubMed.
- M. A. Taylor, J. Janousek, V. Daria, J. Knittel, B. Hage, H.-A. Bachor and W. P. Bowen, Nat. Photonics, 2013, 7, 229 CrossRef CAS.
- S. C. Weber, A. J. Spakowitz and J. A. Theriot, Phys. Rev. Lett., 2010, 104, 238102 CrossRef.
- M. Weiss, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 88, 010101(R) CrossRef.
- M. Magdziarz, A. Weron, K. Burnecki and J. Klafter, Phys. Rev. Lett., 2013, 103, 180602 CrossRef.
- A. N. Kolmogorov, Dokl. Akad. Nauk SSSR, 1940, 26, 115 Search PubMed (in Russian), English transl. in Selected Works of A. N. Kolmogorov, vol. I, Mechanics and Mathematics, ed. V. M. Tikhomirov, Kluwer, Dordrecht, 1991, pp. 303–307 Search PubMed.
- B. B. Mandelbrot and J. W. van Ness, SIAM Rev., 1968, 10, 422 CrossRef.
- I. Goychuk, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2009, 80, 046125 CrossRef.
- K. G. Wang and M. Tokuyama, Physica A, 1999, 265, 341 CrossRef.
- I. Goychuk and P. Hänggi, Phys. Rev. Lett., 2007, 99, 200601 CrossRef CAS.
- R. Zwanzig, J. Stat. Phys., 1973, 9, 215 CrossRef.
- I. Goychuk, Adv. Chem. Phys., 2012, 150, 187 CrossRef CAS.
- R. Zwanzig, Nonequilibrium statistical mechanics, Oxford University Press, Oxford, 2001 Search PubMed.
- R. Kubo, M. Toda and M. Hashitsume, Nonequilibrium statistical mechanics, Springer, Berlin, 2nd edn, 1991 Search PubMed.
- R. J. Ellis and A. P. Minton, Nature, 2003, 425, 27 CrossRef CAS PubMed.
- S. R. McGuffee and A. H. Elcock, PLoS Comput. Biol., 2010, 6, e1000694 Search PubMed.
- N. Hirokawa and R. Takemura, Nat. Rev. Neurosci., 2005, 6, 201 CrossRef CAS PubMed.
- A. T. Jones, M. Gumbleton and R. Duncan, Adv. Drug Delivery Rev., 2003, 55, 1353 CrossRef CAS PubMed.
- J.-F. Chauwin, A. Ajdari and J. Prost, Europhys. Lett., 1994, 27, 421 CrossRef CAS.
- F. Jülicher, A. Ajdari and J. Prost, Rev. Mod. Phys., 1997, 69, 1269 CrossRef.
- F. Jülicher, in Transport and Structure: Their Competitive Roles in Biophysics and Chemistry, Lect. Not. Phys., ed. S. C. Müller, J. Parisi and W. Zimmermann, Springer, Berlin, 1999, vol. 532, p. 46 Search PubMed.
- R. D. Astumian, Science, 1997, 276, 917 CrossRef CAS.
- R. D. Astumian and M. Bier, Biophys. J., 1996, 70, 637 CrossRef CAS.
- M. E. Fisher and A. B. Kolomeisky, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 7748 CrossRef CAS PubMed.
- R. Perez-Carrasco and J. M. Sancho, Biophys. J., 2010, 98, 2591 CrossRef CAS PubMed.
- P. Reimann, Phys. Rep., 2002, 361, 57 CrossRef CAS.
- I. Goychuk, Chem. Phys., 2010, 375, 450 CrossRef CAS PubMed.
- I. Goychuk and V. Kharchenko, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2012, 85, 051131 CrossRef.
- V. Kharchenko and I. Goychuk, New J. Phys., 2012, 14, 043042 CrossRef.
- I. Goychuk and V. Kharchenko, Math. Modell. Nat. Phenom., 2013, 8, 144 CrossRef PubMed.
- V. Kharchenko and I. Goychuk, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 87, 052119 CrossRef.
- Yu. A. Makhnovskii, V. M. Rozenbaum, D.-Y. Yang, S. H. Lin and T. Y. Tsong, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2004, 69, 021102 CrossRef.
- I. Goychuk, V. Kharchenko and R. Metzler, PLoS One, 2014, 9, e91700 Search PubMed.
- K. B. Zeldovich, J.-F. Joanny and J. Prost, Eur. Phys. J. E: Soft Matter Biol. Phys., 2005, 17, 155 CrossRef CAS PubMed.
- H. Kojima, E. Muto, H. Higuchi and T. Yanagida, Biophys. J., 1996, 73, 2012 CrossRef.
- T. G. Mason and D. A. Weitz, Phys. Rev. Lett., 1995, 74, 1250 CrossRef CAS.
- T. A. Waigh, Rep. Prog. Phys., 2005, 68, 685 CrossRef.
- R. A. L. Jones, Soft Condensed Matter, Oxford University Press, Oxford, 2009 Search PubMed.
- A. Gemant, Physics, 1936, 7, 311 CrossRef PubMed.
- J. C. Maxwell, Philos. Trans. R. Soc. London, 1867, 157, 49 CrossRef.
- U. Weiss, Quantum Dissipative Systems, World Scientific, Singapore, 2nd edn, 1999 Search PubMed.
- L. Bruno, V. Levi, M. Brunstein and M. A. Desposito, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2009, 80, 011912 CrossRef CAS.
- A. W. Harrison, D. A. Kenwright, T. A. Waigh, P. G. Woodman and V. J. Allan, Phys. Biol., 2013, 10, 036002 CrossRef PubMed.
- R. G. Palmer, D. L. Stein, E. Abrahams and P. W. Anderson, Phys. Rev. Lett., 1984, 53, 958 CrossRef.
- K. Sekimoto, J. Phys. Soc. Jpn., 1997, 66, 1234 CrossRef CAS.
- I. Derenyi, M. Bier and R. D. Astumian, Phys. Rev. Lett., 1999, 83, 903 CrossRef CAS.
- D. Suzuki and T. Munakata, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2003, 68, 021906 CrossRef.
- H. Wang and G. Oster, Europhys. Lett., 2002, 57, 134 CrossRef CAS.
- J. Wyman, Proc. Natl. Acad. Sci. U. S. A., 1975, 72, 3983 CrossRef CAS.
- T. C. Gard, Introduction to Stochastic Differential Equations, Dekker, New York, 1988 Search PubMed.
- W. Deng and E. Barkai, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2009, 79, 011112 CrossRef.
- J.-H. Jeon and R. Metzler, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2012, 85, 021147 CrossRef.
- B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts and P. Walter, Molecular Biology of the Cell, Garland Science, New York, 4th edn, 2002 Search PubMed.
| This journal is © the Owner Societies 2014 |