Cation-exchanged SAPO-34 for adsorption-based hydrocarbon separations: predictions from dispersion-corrected DFT calculations†
Abstract
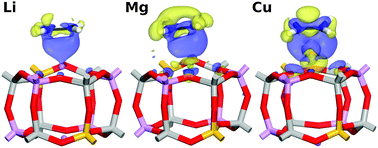
The influence of the nature of the cation on the interaction of the silicoaluminophosphate SAPO-34 with small hydrocarbons (ethane, ethylene, acetylene, propane, propylene) is investigated using periodic density-functional theory calculations including a semi-empirical dispersion correction (DFT-D). Initial calculations are used to evaluate which of the guest-accessible cation sites in the chabazite-type structure is energetically preferred for a set of ten cations, which comprises four alkali metals (Li+, Na+, K+, Rb+), three alkaline earth metals (Mg2+, Ca2+, Sr2+), and three transition metals (Cu+, Ag+, Fe2+). All eight cations that are likely to be found at the SII site (centre of a six-ring) are then included in the following investigation, which studies the interaction with the hydrocarbon guest molecules. In addition to the interaction energies, some trends and peculiarities regarding the adsorption geometries are analysed, and electron density difference plots obtained from the calculations are used to gain insights into the dominant interaction types. In addition to dispersion interactions, electrostatic and polarisation effects dominate for the main group cations, whereas significant orbital interactions are observed for unsaturated hydrocarbons interacting with transition metal (TM) cations. The differences between the interaction energies obtained for pairs of hydrocarbons of interest (such as ethylene–ethane and propylene–propane) deliver some qualitative insights: if this energy difference is large, it can be expected that the material will exhibit a high selectivity in the adsorption-based separation of alkene–alkane mixtures, which constitutes a problem of considerable industrial relevance. While the calculations show that TM-exchanged SAPO-34 materials are likely to exhibit a very high preference for alkenes over alkanes, the strong interaction may render an application in industrial processes impractical due to the large amount of energy required for regeneration. In this respect, SAPOs exchanged with alkaline earth cations could provide a better balance between selectivity and energy cost of regeneration.
- This article is part of the themed collection: High performance computing in the chemistry of materials

 Please wait while we load your content...
Please wait while we load your content...