Study of structural and dynamic characteristics of copper(ii) amino acid complexes in solutions by combined EPR and NMR relaxation methods†
Abstract
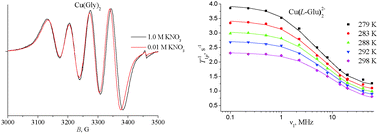
Structural features and dynamical behaviour of the copper(II) bis-complexes with glycine, D-alanine, D-valine, L-serine, L-aspartic acid, L-glutamic acid, L-lysine, L-proline, and sarcosine were studied by combined EPR and NMR relaxation methods. The cis and trans isomers were unambiguously assigned and characterized by EPR data. It was found that addition of a salt background has an influence on the cis–trans isomer equilibrium in favour of the formation of the cis isomer. By comparison of NMRD, DFT computations, and structural data it was shown that only one water molecule is coordinated in the axial position of these complexes. The increased exchange rates of this molecule found for Cu(L-Asp)22−, Cu(L-Glu)22−, Cu(L-LysH)22+, and Cu(L-Pro)2 were attributed to its pushing out by side chain groups of the ligands. By simulation of NMRD profiles an increase of lifetimes of the copper(II) 2nd coordination sphere water molecules was revealed in the presence of additional carboxylic, alcoholic, or ammonium groups of the ligands, as well as the pyrrolidine ring of proline. The very short lifetimes of the 2nd coordination sphere water molecules (4–13 ps at 298 K) were explained in terms of the Frank–Wen structural model by the existence of cavities which draw in quickly enough water molecules from the 2nd coordination sphere.

 Please wait while we load your content...
Please wait while we load your content...