Open Access Article
Open Access ArticleEffect of post-treatments on the photocatalytic activity of Sm2Ti2S2O5 for the hydrogen evolution reaction
Wen
Zhao
a,
Kazuhiko
Maeda†
ab,
Fuxiang
Zhang‡
a,
Takashi
Hisatomi
a and
Kazunari
Domen
*a
aDepartment of Chemical System Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: domen@chemsys.t.u-tokyo.ac.jp
bPrecursory Research for Embryonic Science and Technology (PRESTO), Japan Science and Technology Agency (JST), 4-1-8 Honcho Kawaguchi, Saitama 332-0012, Japan
First published on 6th December 2013
Abstract
The oxysulphide photocatalyst Sm2Ti2S2O5 was synthesized by sulphurizing an amorphous Sm2Ti2O7 prepared using a polymerized complex method under a H2S flow and was used as a H2 evolution photocatalyst in the sacrificial H2 evolution and Z-scheme water splitting reactions. The H2 evolution activity of Rh-loaded Sm2Ti2S2O5 was improved by annealing with sulphur powder and etching with nitric acid. Characterization using XRD, SEM, DRS and XPS suggested that annealing with sulphur decreased the density of the reduced Ti species in Sm2Ti2S2O5 and etching with nitric acid removed the amorphous phases and excessive sulphur species on the surface. After the two post-treatments, platinized Sm2Ti2S2O5 combined with rutile-type TiO2 and NaI showed activity for Z-scheme water splitting under UV irradiation. Although UV irradiation was necessary owing to the use of TiO2, this work provided the first evidence that an oxysulphide photocatalyst was applicable to Z-scheme water splitting.
Introduction
Photocatalytic water splitting has attracted worldwide interest as a promising method for conversion of solar energy into chemical fuels.1–4 In order to utilize solar energy effectively, a photocatalyst must harvest visible light of up to 600 nm or even longer.2Z-scheme water splitting systems based on two-step photoexcitation are typically composed of a H2 evolution photocatalyst, an O2 evolution photocatalyst, and electron mediators.3,4 During photoexcitation, electrons reduce H+ to H2 and holes oxidize the reduced form of redox mediators on the H2 evolution photocatalyst, while on the O2 evolution photocatalyst, electrons reduce the oxidized form of redox mediators and holes oxidize H2O to O2. It has also been found that Z-scheme water splitting can complete even in the absence of redox couples owing to the interparticle electron transfer, where holes in a H2 evolution photocatalyst and electrons in an O2 evolution photocatalyst recombine via physical contact.4,5 Compared to the one-step excitation mechanism that uses a single visible-light-responsive photocatalyst, the two-step excitation mechanism has several advantages:6 it enables the use of a semiconductor that is active for either water reduction or oxidation alone as long as it is active for the oxidation or reduction of the redox mediators, respectively. Because the energy required to drive either photocatalyst can be lower, a wider range of visible-light-active photocatalysts are applicable. For example, WO3, BiVO4, and Rh-doped SrTiO3 have been employed in Z-scheme water splitting although they are active only for H2 or O2 evolution in the presence of appropriate sacrificial reagents.6–8 Photocatalysts that are capable of both the H2 and O2 evolution reactions with sacrificial reagents are also applicable to Z-scheme water splitting, as has been demonstrated with TaON6,9 and Ta3N5.10 Moreover, H2 and O2 are generated on different photocatalysts, which could in principle offer a simple means of separating the gaseous products using an appropriate membrane filter.8 Much effort has been devoted to construction of efficient Z-scheme systems by developing new materials and efficient electron relays.Sm2Ti2S2O5, an oxysulphide semiconductor photocatalyst, has a crystal structure similar to that of a Ruddlesden–Popper-type layered perovskite oxide.11 Under visible light irradiation (λ < 650 nm), Sm2Ti2S2O5 can evolve H2 or O2 from aqueous solutions containing sacrificial electron donors (Na2S–Na2SO3 or methanol) or acceptors (Ag+), respectively.11 This indicates that Sm2Ti2S2O5 has band positions suitable for water splitting under visible light irradiation. However, Sm2Ti2S2O5 and other oxysulphides have not been applied to Z-scheme water splitting successfully. Earlier studies on the photocatalyst Sm2Ti2S2O5 involved the H2S gas sulphurization method for the preparation of fine Sm2Ti2S2O5 particles,12 and the loading and addition of various metal species to Sm2Ti2S2O5.13–15 It was found that promoting both the photocatalytic reduction of water and the oxidation of sacrificial electron donors as well as reducing the defect density of Sm2Ti2S2O5 were important for improving the photocatalytic H2 evolution rate on Sm2Ti2S2O5 from an aqueous solution of Na2S and Na2SO3. In this study, methods of modifying Sm2Ti2S2O5 were investigated to improve the H2 evolution activity. After appropriate post-treatments such as annealing with sulphur and etching with nitric acid, Sm2Ti2S2O5 loaded with Rh exhibited a higher activity for the sacrificial H2 evolution reaction. The modified Sm2Ti2S2O5 was used as a H2 evolution photocatalyst in Z-scheme water splitting. It was found that Pt-loaded Sm2Ti2S2O5 suspended with TiO2 (rutile) in an aqueous NaI solution was capable of splitting water into H2 and O2 under UV irradiation (λ > 300 nm).
Experimental
Amorphous Sm2Ti2O7 was synthesized as an oxide precursor by a polymerized complex method.12 Titanium tetraisopropoxide (0.02 mol, Kanto Chemical Co., 97%) and anhydrous citric acid (0.3 mol, Wako Pure Chemicals, 98%) were dissolved in ethylene glycol (0.2 mol, Kanto Chemical Co., 99.5%) at room temperature, and the mixture was heated at 333 K until the reagents were completely dissolved. Subsequently, Sm(NO3)3·6H2O (0.02 mol, Wako Pure Chemicals Co., 99.5%) and 20 mL methanol were added to the solution. The mixture was stirred at 403 K until a transparent gel was formed. It was polymerized and carbonized at 523, 573, and 623 K for 1 h each and finally calcined at 773 K for 12 h to remove the carbon completely. The resulting amorphous oxide was sulphurized at 1223 K for 1 h under a flow of H2S (10 mL min−1) and calcined for 2 h in air at 573 K.As-synthesized Sm2Ti2S2O5 was sealed in evacuated quartz tubes with 5 wt% sulphur powder (High Purity Chemicals Co., 99.99%) with respect to Sm2Ti2S2O5 and annealed at 1223 K for 20 h.12 Nitric acid treatment was conducted by dispersing Sm2Ti2S2O5 powder (ca. 0.5 g) in HNO3 (Wako Pure Chemicals Co., 69.8 wt%) for 10 min at room temperature, followed by rinsing with distilled water, filtration, drying at 343 K, and heat treatment at 573 K for 1 h in air. The mass loss during the whole nitric acid etching procedure was approximately 40%, while the mass loss during the rinsing and filtration procedures was approximately 8%. When both treatments were carried out, the nitric acid etching was performed after the sulphur annealing. Sm2Ti2S2O5 samples subjected to the sulphur annealing alone, to the nitric acid etching alone, and to both procedures will hereafter be denoted as Sm2Ti2S2O5 (S), Sm2Ti2S2O5 (HNO3), and Sm2Ti2S2O5 (S + HNO3), respectively.
The samples were characterized using X-ray powder diffraction (XRD; RINT-Ultima III, Rigaku; Cu Kα), ultraviolet-visible diffuse-reflectance spectroscopy (DRS; V-670, JASCO), field-emission scanning electron microscopy (FE-SEM; S-4700, Hitachi) and X-ray photoelectron spectroscopy (XPS; JPS-9000, JEOL). The binding energy for each sample was corrected by the reference C 1s peak (285.0 eV). The peak areas of Sm 3d5/2, Ti 2p, S 2p3/2, and O 1s were acquired by integrating the signals over 1090.0–1077.0, 468.0–455.8, 171.8–157.4, and 535.7–526.5 eV, respectively.
The photocatalytic H2 evolution reactions were carried out in an inner irradiation-type Pyrex reaction vessel connected to an air-tight closed gas circulation system made of Pyrex glass. An aqueous solution (400 mL) containing the photocatalyst sample (0.2 g), Na2S–Na2SO3 (0.2 M each) was irradiated with a 450 W high-pressure Hg lamp through a NaNO2 solution (2 M) as an optical filter, the cutoff wavelength of which was 400 nm. For the H2 evolution reaction, Rh (1.5 wt%) was loaded as a H2 evolution cocatalyst by photodeposition from the Na2S–Na2SO3 aqueous solutions.13,14 A calculated amount of RhCl3·xH2O (Aldrich, 38–40% as Rh) precursor added to the solution was reduced to metallic Rh by photoexcited electrons from the photocatalyst in the initial stage of the reaction. The reactant solution was maintained at room temperature by flowing cooling water during the reaction. The evolved gas was analyzed by gas chromatography (GC, TCD, Shimadzu).
For Z-scheme water splitting, Pt-loaded Sm2Ti2S2O5 (0.2 g), rutile TiO2 (0.2 g), and NaI (50 mM) were used as a H2 evolution photocatalyst, O2 evolution photocatalyst, and redox mediator, respectively. Pt is employed as an effective H2 evolution cocatalyst in Z-scheme water splitting involving the redox couple IO3−/I−.6,10,15–17 Pt (2 wt%) was photodeposited on Sm2Ti2S2O5 from a calculated amount of H2PtCl6·6H2O (Aldrich, 38–40% Pt) in an aqueous NaI solution (pH = 6). The platinized sample was collected and used for Z-scheme water splitting. The pH of the solution containing the H2 evolution photocatalyst, O2 evolution photocatalyst, and redox mediator was adjusted using an aqueous NaOH solution. The solution was evacuated and irradiated with a 450 W high-pressure Hg lamp (λ > 300 nm). Note that conventional TiO2 (rutile) was employed to assess the applicability of Sm2Ti2S2O5 as a H2 evolution photocatalyst in Z-scheme water splitting, since rutile-type TiO2 worked effectively as an O2 evolution photocatalyst in Z-scheme systems involving IO3−/I− owing to preferential adsorption of IO3− ions onto rutile-type TiO2 and its sufficient activity of for IO3− reduction even without elaborate modifications while necessitating UV illumination.17
Results and discussion
Effects of post-treatments
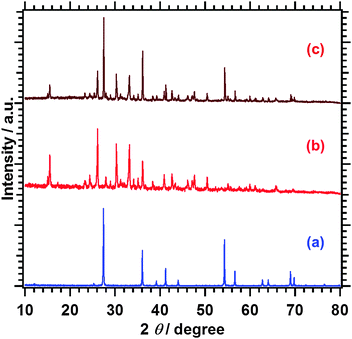
Fig. 1 shows XRD patterns of the Sm2Ti2S2O5 samples before and after the post-treatments. The diffraction profile of the Sm2Ti2S2O5 phase was unchanged and no impurity phase was generated by the post-treatments. The peak intensities of Sm2Ti2S2O5 and Sm2Ti2S2O5 (HNO3) were identical, while those of Sm2Ti2S2O5 (S) and Sm2Ti2S2O5 (S + HNO3) became somewhat stronger. This suggests that the sulphur annealing at 1223 K improved the crystallinity of Sm2Ti2S2O5 and that the nitric acid etching did not change the bulk crystallinity of Sm2Ti2S2O5.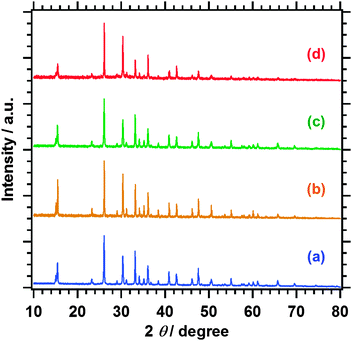 | ||
| Fig. 1 XRD patterns of (a) Sm2Ti2S2O5, (b) Sm2Ti2S2O5 (S), (c) Sm2Ti2S2O5 (HNO3), and (d) Sm2Ti2S2O5 (S + HNO3). | ||
Fig. 2 shows SEM images of the corresponding Sm2Ti2S2O5 samples. Sm2Ti2S2O5 consisted of plate-like submicron particles, which formed massive secondary particles several micrometers in size. During the sulphur annealing, these aggregates were sintered into 0.5–1 μm particles with smoother surfaces. When the samples were etched with HNO3, dramatic changes were observed in the surface morphologies of Sm2Ti2S2O5 and Sm2Ti2S2O5 (S). It appeared that the outer surface of the samples had dissolved, exposing the interior consisting of submicron Sm2Ti2S2O5 grains. This verified the speculation of our previous study that the large Sm2Ti2S2O5 (S) particles were polycrystalline.12 The Sm2Ti2S2O5 (S + HNO3) grains were, on average, larger than the Sm2Ti2S2O5 (HNO3) grains.
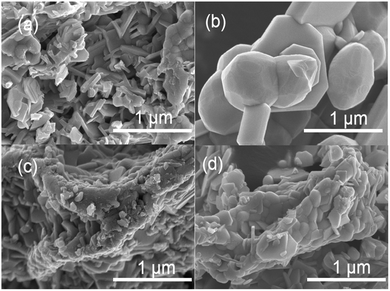 | ||
| Fig. 2 SEM images of (a) Sm2Ti2S2O5, (b) Sm2Ti2S2O5 (S), (c) Sm2Ti2S2O5 (HNO3), and (d) Sm2Ti2S2O5 (S + HNO3). | ||
The surface compositions of the Sm2Ti2S2O5 samples before and after the post-treatments are tabulated in Table 1. Pristine Sm2Ti2S2O5 nearly exhibited the stoichiometric Sm/Ti ratio, while the S/Ti and O/Ti ratios were larger than the corresponding stoichiometric ratios. We obtained similar results in our previous study.12 This is because the Sm2Ti2S2O5 surface was enriched with elemental and oxidized sulphur species when Sm2Ti2S2O5 was sulphurized under a H2S flow and annealed in air. Sm2Ti2S2O5 (HNO3) after the nitric acid etching exhibited a smaller Sm/Ti ratio than pristine Sm2Ti2S2O5. This indicates that the Sm2Ti2S2O5 surface was indeed dissolved by nitric acid, particularly the Sm component with weak basicity. The sulphur annealing did not change the surface Sm/Ti ratio significantly, while generating excessive sulphur on the surface of Sm2Ti2S2O5 (S). Sm2Ti2S2O5 (S + HNO3) maintained the Sm/Ti ratio of Sm2Ti2S2O5 (S), unlike the case of Sm2Ti2S2O5 (HNO3) after the nitric acid treatment. It is thought that a part of the pristine Sm2Ti2S2O5 sample was rather amorphous and thus the nitric acid etching resulted in preferential dissolution of Sm species. However, annealing in the presence of sulphur improved the crystallinity of Sm2Ti2S2O5, incorporating amorphous Sm species into the crystal structure, thereby suppressing the leaching of Sm species during the nitric acid treatment. Note that the surface of Sm2Ti2S2O5 (S) was still etched, as was evident from the SEM images. This resulted in the partial removal of the excess sulphur species on the surface.
| Sample | Sm/Ti | S/Ti | O/Ti |
|---|---|---|---|
| Sm2Ti2S2O5 | 1.1 | 1.9 | 6.6 |
| Sm2Ti2S2O5 (HNO3) | 0.6 | 2.2 | 5.8 |
| Sm2Ti2S2O5 (S) | 0.9 | 2.6 | 7.5 |
| Sm2Ti2S2O5 (S + HNO3) | 0.9 | 2.0 | 6.5 |
The DRS shown in Fig. 3 confirms that the pristine sample exhibited a light absorption onset at approximately 650 nm, which corresponded to the band gap transition of Sm2Ti2S2O5.11 The background absorption was also observed, presumably because of reduced Ti species. The reduced Ti species may have been generated by the exposure to heated H2S.12 Sm2Ti2S2O5 (HNO3) had a similar absorption onset and background absorption. The fact that etching of the surface did not weaken the background absorption indicates that the reduced Ti species were present in the bulk of the sample. Sm2Ti2S2O5 (S) and Sm2Ti2S2O5 (S + HNO3), which were subjected to the sulphur annealing treatment, had similar absorption onsets but lower background absorption. Considering the dramatic changes in morphology that occurred during the sulphur annealing, it is reasonable to assume that the reduced Ti species in the bulk were oxidized by elemental sulphur upon annealing at 1223 K.
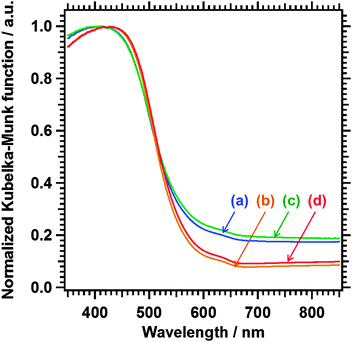 | ||
| Fig. 3 UV-vis DRS of (a) Sm2Ti2S2O5, (b) Sm2Ti2S2O5 (S), (c) Sm2Ti2S2O5 (HNO3), and (d) Sm2Ti2S2O5 (S + HNO3). | ||
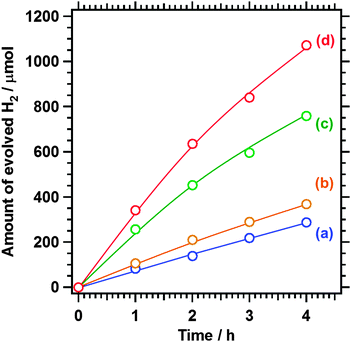
Fig. 4 shows the reaction time courses of photocatalytic H2 evolution using pristine and post-treated Sm2Ti2S2O5 samples. The photocatalytic activity of Sm2Ti2S2O5 was improved by the sulphur annealing and the nitric acid etching, and combining these post-treatments resulted in the highest activity. During the sulphurization of amorphous Sm2Ti2O7 to Sm2Ti2S2O5, a certain amount of Ti3+ species was generated because of the reduction of Ti4+ under the heated H2S atmosphere. The generation of reduced Ti species is accompanied by the formation of anion vacancies. These vacancies tend to act as recombination centres for photoexcited electrons and holes and suppress the photocatalytic activity.12,13 The sulphur annealing can potentially produce a higher activity owing to the lower density of reduced Ti species in Sm2Ti2S2O5. In reality, however, hardly any activity enhancement was observed upon sulphur annealing. This is likely due to the presence of excess sulphur species on the surface, which blocked the surface active sites and thus prevented the deposition of Rh as H2 evolution sites.12 The sintering of Sm2Ti2S2O5 may also have led to the lower-than-expected photocatalytic activity.12 The nitric acid etching improved the H2 evolution activity by a factor of three although the Sm component was deficient on the sample surface. The SEM and XPS results suggest that the enhanced activity of Sm2Ti2S2O5 (HNO3) is attributable to the elimination of the defective amorphous layers on the surface that would otherwise have prevented charge transfer. Sm2Ti2S2O5 (S + HNO3) benefited from both post-treatments. Moreover, the excess sulphur species on Sm2Ti2S2O5 (S) were eliminated in part by the nitric acid etching, and the Sm deficiency on the surface observed in the case of Sm2Ti2S2O5 (HNO3) was avoided owing to the improved crystallinity. As a result, Sm2Ti2S2O5 (S + HNO3) showed a H2 evolution rate that was 4.5 times higher than that of pristine Sm2Ti2S2O5.
Application of Sm2Ti2S2O5 to Z-scheme water splitting
Table 2 shows the amounts of H2 and O2 evolved in Z-scheme water splitting reactions over 5 h using Pt-loaded Sm2Ti2S2O5 samples as a H2 evolution photocatalyst. Simultaneous evolution of H2 and O2 was observed only when Sm2Ti2S2O5 (S + HNO3) was used as a H2 evolution photocatalyst together with TiO2 (rutile) as an O2 evolution photocatalyst and NaI as a redox mediator. The ratio of H2 to O2 produced was higher than the stoichiometry of overall water splitting because of the presence of excess electron donors (NaI) in the beginning of the reaction. When one of the components, i.e., the H2 evolution photocatalyst, O2 evolution photocatalyst, or redox mediator, was missing, the gas evolution was negligible. This confirms that the water splitting reaction occurred via the Z-scheme mechanism.| H2 evolution photocatalystb | O2 evolution photocatalyst | Redox mediator | Activityc/μmol | |
|---|---|---|---|---|
| H2 | O2 | |||
| a Reaction conditions: photocatalysts, 0.2 g each; reaction solution, aqueous NaI solution (430 mL, 50 mM, pH = 12); light source, 450 W high-pressure Hg lamp. b Loaded with 2 wt% of Pt by photodeposition. c Estimated from the amount of gases evolved in 5 h. | ||||
| Sm2Ti2S2O5 | TiO2 (rutile) | NaI | 10 | 0 |
| Sm2Ti2S2O5 (S) | TiO2 (rutile) | NaI | 13 | 0 |
| Sm2Ti2S2O5 (HNO3) | TiO2 (rutile) | NaI | 11 | 0 |
| Sm2Ti2S2O5 (S + HNO3) | TiO2 (rutile) | NaI | 45 | 16 |
| None | TiO2 (rutile) | NaI | 0 | 0 |
| Sm2Ti2S2O5 (S + HNO3) | None | NaI | 0.6 | 0 |
| Sm2Ti2S2O5 (S + HNO3) | TiO2 (rutile) | None | 0 | 0 |
The use of pristine Sm2Ti2S2O5, Sm2Ti2S2O5 (S), and Sm2Ti2S2O5 (HNO3) did not lead to successful O2 evolution, while producing a certain amount of H2. In addition, the H2 evolution ceased during the initial stage of the reaction. In Z-scheme water splitting in the presence of reversible redox couples, simultaneous evolution of H2 and O2 is often difficult because of backward reactions on the respective photocatalysts. IO3− produced by the oxidation of I− on Sm2Ti2S2O5 could largely be reduced back to I− unless the H2 evolution activity was high enough to outweigh the thermodynamically favorable backward reaction. In addition, the oxygen evolution on rutile-type TiO2 could be suppressed by the oxidation of I− when the concentration of I− was high.17 The absence of O2 evolution thus suggests that the activities of the above three Sm2Ti2S2O5 samples for H2 evolution and I− oxidation were too low to provide a sufficient amount of IO3− in the reaction solution. Further characterization is necessary to determine why, among the Sm2Ti2S2O5 samples subjected to different post-treatments, only Sm2Ti2S2O5 (S + HNO3) was active for Z-scheme water splitting. However, the present results suffice to show that the appropriate post-treatments enabled the oxysulphide photocatalyst to be used as a H2 evolution photocatalyst for Z-scheme water splitting and that lowering the densities of both reduced Ti species and surface impurities was necessary.
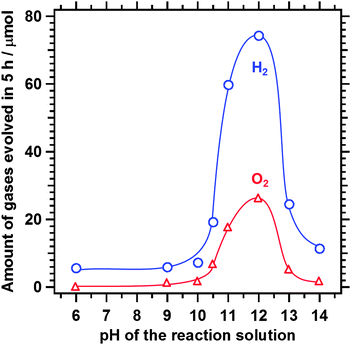
Fig. 5 shows the dependence of the photocatalytic activity of the Z-scheme system for water splitting on the pH of the reaction solution. Z-scheme water splitting was enhanced significantly as the pH increased from 6 to 12 and was suppressed at pH > 12. O2 evolution was almost negligible at pH < 9. This is because the main oxidative product on Pt-loaded Sm2Ti2S2O5 (S + HNO3) was I3−, which failed to serve as an efficient electron acceptor for O2 evolution over rutile TiO2. The photocatalytic activity decreased at pH > 12. This is probably because of the decreasing driving force of Sm2Ti2S2O5 for H2 evolution with increasing pH. It has been found previously that the potential of the band gap of Sm2Ti2S2O5 was independent of the pH of the solution,11 while the H2 evolution potential shifted negatively with increasing pH. Thus, a basic solution at a pH of around 11–12 was the most favourable for Z-scheme water splitting using Sm2Ti2S2O5, TiO2, and I−/IO3−.
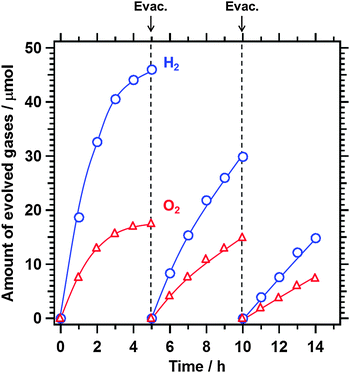
Fig. 6 shows the results of Z-scheme water splitting with intermittent evacuations. The ratio of H2 and O2 approached 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 after the first five-hour reaction and subsequent evacuation as the excess I− was converted into IO3− during the reaction. The rate of gas evolution gradually decreased as the gas product accumulated in the closed system because the backward reaction from H2 and O2 to H2O occurred on the Pt used as a cocatalyst. The rate of water splitting recovered to some extent upon evacuation. However, the photocatalytic activity of the system gradually decreased. XRD patterns presented in Fig. 7 show that the sample after the reaction was a mixture of Sm2Ti2S2O5 (S + HNO3) and rutile-type TiO2. This result suggests that the bulk of Sm2Ti2S2O5 (S + HNO3) was mostly stable during the water splitting reaction. The deactivation may be due to the photo-oxidation of the surface of Sm2Ti2S2O5 by photoexcited holes under the intense UV illumination. Although UV illumination and stabilization of Sm2Ti2S2O5 were still necessary, this represents the first instance of Z-scheme water splitting achieved using an oxysulphide photocatalyst as a H2 evolution photocatalyst. It is expected that dual cocatalyst loading on Sm2Ti2S2O518,19 and the use of visible-light-sensitive O2 evolution photocatalysts will allow stable water splitting under visible light illumination.
1 after the first five-hour reaction and subsequent evacuation as the excess I− was converted into IO3− during the reaction. The rate of gas evolution gradually decreased as the gas product accumulated in the closed system because the backward reaction from H2 and O2 to H2O occurred on the Pt used as a cocatalyst. The rate of water splitting recovered to some extent upon evacuation. However, the photocatalytic activity of the system gradually decreased. XRD patterns presented in Fig. 7 show that the sample after the reaction was a mixture of Sm2Ti2S2O5 (S + HNO3) and rutile-type TiO2. This result suggests that the bulk of Sm2Ti2S2O5 (S + HNO3) was mostly stable during the water splitting reaction. The deactivation may be due to the photo-oxidation of the surface of Sm2Ti2S2O5 by photoexcited holes under the intense UV illumination. Although UV illumination and stabilization of Sm2Ti2S2O5 were still necessary, this represents the first instance of Z-scheme water splitting achieved using an oxysulphide photocatalyst as a H2 evolution photocatalyst. It is expected that dual cocatalyst loading on Sm2Ti2S2O518,19 and the use of visible-light-sensitive O2 evolution photocatalysts will allow stable water splitting under visible light illumination.
 | ||
| Fig. 7 XRD patterns of (a) rutile-type TiO2, (b) Sm2Ti2S2O5 (S + HNO3), and (c) after the Z-scheme water splitting reaction with rutile-type TiO2 at pH 11. | ||
Conclusions
The processes of annealing with sulphur and etching with nitric acid were studied to improve the photocatalytic activity of Sm2Ti2S2O5 for H2 evolution in sacrificial water reduction and Z-scheme water splitting. The photocatalytic activity improved by a factor of 4.5 when the sulphur annealing and nitric acid etching were applied in combination. The effects of these post-treatments were attributed to the oxidation of reduced Ti3+ species and the removal of amorphous surface phases and excess sulphur species, respectively.Following the sulphur annealing and the nitric acid etching, Sm2Ti2S2O5 could be used as a H2 evolution photocatalyst in Z-scheme water splitting in combination with TiO2 as an O2 evolution photocatalyst and NaI as a redox mediator. All three of the components were necessary to drive Z-scheme water splitting. Employing both post-treatments was essential for successful Z-scheme water splitting, which indicated the importance of removing reduced Ti3+ species and surface impurities from Sm2Ti2S2O5. Although UV irradiation was necessary because of the use of TiO2, the present study revealed that appropriate modifications allowed an oxysulphide photocatalyst to serve as a H2 evolution photocatalyst in Z-scheme water splitting.
Acknowledgements
This work was financially supported by the Grant-in-Aid for Specially Promoted Research (no. 23000009) of the Japan Society for the Promotion of Science (JSPS) and the Advanced Low Carbon Technology Research and Development Program (ALCA) of the Japan Science and Technology Agency (JST). Further financial support was provided by JSPS through the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST), initiated by the Council for Science and Technology Policy (CSTP), and by an international exchange program of the A3 Foresight Program of JSPS. Z.W. gratefully acknowledges Dr Curulla-Ferre, Prof. Wimmer, and Dr Yagi of TOTAL S.A. for scientific discussions and financial support. K. M. is indebted to the Nippon Sheet Glass Foundation for Materials Science and Engineering for funding support.Notes and references
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253 RSC.
- K. Maeda and K. Domen, J. Phys. Chem. Lett., 2010, 2655 CrossRef CAS.
- R. Abe, J. Photochem. Photobiol., C, 2010, 11, 179 CrossRef CAS PubMed.
- K. Maeda, ACS Catal., 2013, 3, 1486 CrossRef CAS.
- Y. Sasaki, H. Nemoto, K. Saito and A. Kudo, J. Phys. Chem. C, 2009, 113, 17536 CAS.
- K. Maeda, M. Higashi, D. Lu, R. Abe and K. Domen, J. Am. Chem. Soc., 2010, 132, 5858 CrossRef CAS PubMed.
- Y. Sasaki, A. Iwase, H. Kato and A. Kudo, J. Catal., 2008, 259, 133 CrossRef CAS PubMed.
- Y. Sasaki, H. Kato and A. Kudo, J. Am. Chem. Soc., 2013, 135, 5441 CrossRef CAS PubMed.
- M. Higashi, R. Abe, A. Ishikawa, T. Takata, B. Ohtani and K. Domen, Chem. Lett., 2008, 37, 138 CrossRef CAS.
- M. Tabata, K. Maeda, M. Higashi, D. Lu, T. Takata, R. Abe and K. Domen, Langmuir, 2010, 26, 9161 CrossRef CAS PubMed.
- A. Ishikawa, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, J. Am. Chem. Soc., 2002, 124, 13547 CrossRef CAS PubMed.
- A. Ishikawa, Y. Yamada, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, Chem. Mater., 2003, 15, 4442 CrossRef CAS.
- F. Zhang, K. Maeda, T. Takata and K. Domen, J. Catal., 2011, 280, 1 CrossRef CAS PubMed.
- F. Zhang, K. Maeda, T. Takata, T. Hisatomi and K. Domen, Catal. Today, 2012, 185, 253 CrossRef CAS PubMed.
- K. Sayama, K. Mukasa, R. Abe, Y. Abe and H. Arakawa, Chem. Commun., 2001, 2416 RSC.
- K. Sayama, K. Mukasa, R. Abe, Y. Abe and H. Arakawa, J. Photochem. Photobiol., A, 2002, 148, 71 CrossRef CAS.
- R. Abe, K. Sayama and H. Sugihara, J. Phys. Chem. B, 2005, 109, 16052 CrossRef CAS PubMed.
- F. Zhang, K. Maeda, T. Takata and K. Domen, Chem. Commun., 2010, 46, 7313 RSC.
- R. Li, Z. Chen, W. Zhao, F. Zhang, K. Maeda, B. Huang, S. Shen, K. Domen and C. Li, J. Phys. Chem. C, 2013, 117, 376 CAS.
Footnotes |
| † Current address: Department of Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, 2-12-1-NE-2 Ookayama, Meguro-ku, Tokyo 152-8550, Japan. |
| ‡ Current address: State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Zhongshan Road 457, Dalian 116023, China. |
| This journal is © the Owner Societies 2014 |