Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Li ion dynamics in TiO2 anode materials with an ordered hierarchical pore structure – insights from ex situ NMR
Patrick
Bottke
*a,
Yu
Ren
b,
Ilie
Hanzu
a,
Peter G.
Bruce
b and
Martin
Wilkening
a
aDFG Research Unit 1277 “Mobility of Lithium Ions in Solids” (TP 7), and Institute for Chemistry and Technology of Materials, Graz University of Technology Stremayrgasse 9, A-8010, Graz, Österreich. E-mail: bottke@tugraz.at
bSchool of Chemistry, University of St Andrews The Purdie Building, North Haugh, St Andrews, Fife KY16 9ST, UK
First published on 11th December 2013
Abstract
Ex situ Nuclear Magnetic Resonance (NMR) measurements were carried out to study lithium ion dynamics in lithium intercalated mesoporous anatase (LixTiO2) serving as an anode material for rechargeable lithium-ion batteries. As has been shown recently, hierarchically ordered TiO2 shows excellent cycling performance and ensures a high lithium storage capacity. 7Li spin–lattice relaxation NMR and stimulated echo NMR serve as a powerful combination to shed light on the Li hopping processes from an atomic-scale point of view. To determine atomic Li jump rates and microscopic activation energies temperature-variable SLR NMR measurements, in both the laboratory and rotating frame of reference, as well as mixing-time dependent spin-alignment echo NMR measurements were carried out. The results point to moderate Li diffusivities; however, in a lithium-ion cell this is compensated for by taking advantage of nm-structured materials with greatly reduced diffusion lengths. Importantly, although a phase transition from tetragonal symmetry to orthorhombic symmetry takes place at increased states of charge, the diffusion parameters and activation energies probed (0.4 to 0.5 eV) do depend weaker on Li content x than expected. Thus, despite the increased value of x, the evolution of the orthorhombic phase seems to support Li diffusivity rather than to affect the transport properties in a negative way. This interesting feature might be highly beneficial for the excellent cycling behavior observed recently.
1 Introduction
The development of new electrode materials for lithium-ion batteries is a vital topic in materials science.1–6 Currently, materials with nm-sized dimensions,7i.e., those being characterized by large surface areas, are strongly considered to act as powerful anode and cathode materials significantly improving the performance of today's lithium ion batteries.1,8–13 Combined with an ordered 3D pore structure14 such materials ensure high storage capacities and facile Li insertion and removal. This is inter alia related to short diffusion lengths of the nm-sized materials1 as well as to the involvement of Li surface storage8 (or even pseudo-capacitive faradaic processes15,16) due to the large volume fraction of interfacial regions.Quite recently, excellent cycling behavior of a lithium-ion battery was achieved when mesoporous TiO2 with a 3D hierarchical pore structure served as an anode material.14 Even after extensive charging and discharging of the battery, the hierarchical pore structure is preserved throughout clearly illustrating the high stability of the anode material.14 Initially, TiO2 crystallizes with tetragonal symmetry. However, upon Li insertion the crystal structure transforms into an orthorhombic one,14 see also analogous studies where NMR has been used to monitor these changes.17–19 Besides kinetic effects also Li diffusion properties are anticipated to govern the insertion and removal rates. So far, only a few studies can be found in the literature being concerned with the atomic-scale measurement of Li self-diffusion parameters of TiO2-based anode materials with different states of charges.17,20–23 The present study aims at the question if and to which extent Li self-diffusion changes as a function of Li content x in LixTiO2. Since LixTiO2 is a mixed conductor, that is, electrons and lithium ions contribute to the overall conductivity, those methods which are able to solely probe Li ion dynamics, such as NMR, represent favorable techniques for this purpose.17,20,22
Therefore, we used complementary 7Li NMR techniques20,24,25 to study the Li+ self-diffusion parameters such as microscopic activation energies and jump rates. The techniques applied are sensitive to Li motional processes on quite different time scales.26 In particular, while 7Li spin-alignment echo (SAE) NMR27–30 is useful to probe rather slow Li motions with correlation rates lower than 104 Hz, 7Li NMR spin–lattice relaxation (SLR) measurements, in both the laboratory (SLRL) and rotating frame of reference (SLRρ),26,31,32 are applicable to detect hopping processes with rates ranging from 105 Hz to 109 Hz. Interestingly, considering x and the reversible phase transformation mentioned above, the dynamic parameters deduced from NMR, if identified with bulk processes, turned out to be less influenced by the Li content than expected,33 see also ref. 34. In contrast, the orthorhombic structure seems to facilitate Li migration which seems to be beneficial for the performance of the battery.
2 Experimental
Synthesis and characterization of mesoporous anatase are described elsewhere in detail, see ref. 14. Mesoporous anatase was prepared using the silica KIT-6 as a hard template14 and lithium intercalation was carried out electrochemically at a rate of 10 mA g−1. Electrochemical cells were constructed by mixing the active material and Super S carbon (MMM) in the weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and pressed into a pellet. After drying at 80 °C under vacuum for 8 hours, the electrodes were assembled into cells with a Li anode and an LP 30 electrolyte (Merck; 1 M LiPF6 in 1
1 and pressed into a pellet. After drying at 80 °C under vacuum for 8 hours, the electrodes were assembled into cells with a Li anode and an LP 30 electrolyte (Merck; 1 M LiPF6 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ethylene carbonate/dimethyl carbonate).
1 v/v ethylene carbonate/dimethyl carbonate).
The cells were constructed and handled in an Ar-filled MBraun glovebox. The cells were cycled at 10 mA g−1 (C/33). The first discharge was used to prepare the three different samples with overall capacities of 30 mA h g−1, 117 mA h g−1, and 248 mA h g−1 at certain cut-off potentials. This corresponds to Lix=0.09TiO2, Li0.35TiO2, and Li0.74TiO2 respectively (see Fig. 1). After that the electrodes were rinsed with a small amount of dry solvent (dimethyl carbonate) to remove the residual electrolyte. They were then left under dynamic vacuum overnight to ensure that all solvent had evaporated. Prior to the temperature-variable NMR measurements, the samples were fire-sealed in glass ampoules to protect them permanently from any traces of moisture.
7Li NMR data were recorded at temperatures ranging from 153 K to 453 K on MSL 100 and MSL 400 solid-state NMR spectrometers (Bruker). While the MSL 100 (Bruker) is connected to a Kalmus amplifier (400 W) and a field-variable Oxford cryo-magnet with 4.7 T, the MSL 400 (in standard configuration, Bruker) was used in combination with a shimmed Oxford cryo-magnet with a nominal magnetic field of 9.4 T. The corresponding resonance frequencies ω0/2π were 78 and 155 MHz, respectively. Mostly, commercial probes were used to record static NMR line shapes, SLR rates and SAE NMR decay rates as a function of temperature. Typically, 90° pulse lengths were in the order of 4 to 6 μs. The temperature in the sample chamber was monitored using a Ni–CrNi thermocouple connected to an Oxford ITC. The accuracy of temperature adjustment was approximately ±2 K. Temperatures below room temperature were reached by heating a stream of freshly evaporated nitrogen gas. Above room temperature heated air was used to achieve the desired temperatures.
NMR spectra, recorded at 155 MHz, were used to determine the line width (fwhm, full widths at half maximum) for the analysis of so-called motional narrowing curves. The corresponding free induction decays were recorded with the help of a single pulse sequence using a delay of 5 × T1 between each scan to ensure full longitudinal relaxation; T1 denotes the spin–lattice relaxation time. In the case of Lix=0.09TiO2 up to 64 scans were accumulated to compensate for the weak signal-to-noise ratio.
The classical saturation recovery pulse sequence35 has served to determine 7Li SLRL NMR rates. We used a comb of 10 closely spaced 90° pulses to destroy any longitudinal magnetization Mz(t). The subsequent recovery of Mz(t) was then recorded with a 90° detection pulse as a function of delay time and temperature. The transients follow non-exponential time behavior and have been parameterized with stretched exponentials.
Slower Li diffusion processes were measured via the spin-lock technique introduced by Ailion and Slichter:36–38 90°−spin-lock pulse−acquisition (acq.) at angular locking frequencies ω1/2π in the kHz range. Our locking pulses varied from tlock = 1 μs to tlock = 100 ms. As in the case of SLRL the transients Mρ(tlock) of SLRρ NMR can only be described by stretched exponentials. Finally, mixing-time (tm) dependent SAE NMR decay curves were recorded with the help of the Jeener–Broekaert39,40 three-pulse sequence: (90°)X − tp − (45°)Y − tm − 45° − acq. We used a fixed preparation time tp of 10 μs to acquire two-time single-spin (sin–sin) correlation functions. The mixing time was varied from 100 μs up to several seconds, i.e., spanning a time window of many decades. A 32-fold phase cycle39,41 was employed to suppress unwanted coherences and to eliminate dipolar contributions to the echo showing up after the reading pulse. Fourier transformation of the spin-alignment echo, starting from the top of the signal, results in SAE NMR spectra useful to highlight quadrupole intensities due to the interaction of the quadrupole moment of 7Li (spin-3/2 nucleus) and non-vanishing electric field gradients (see below).
3 Results and discussion
Temperature-variable NMR line shape measurements give first insights into the microscopic jump processes of Li ions in solids.42 At low temperature (see Fig. 2), that is, at temperatures for which the mean Li jump rate is much lower than the spectral line width, both the shape and the width of an NMR line are determined by rigid dipolar interactions of the Li spins. With increasing T, however, homonuclear dipole–dipole interactions predominantly governing the line width are increasingly averaged due to Li translational motion on a sufficiently large length scale.43 As a result, the NMR line undergoes a pronounced narrowing process. In many cases, the initial Gaussian shape of the NMR central line turns into a Lorentzian one determining the spectra in the regime of extreme narrowing. This is also observed for the sample with x = 0.74. The spectra shown in Fig. 2 were obtained by analyzing free induction decays. Thus, quadrupolar intensities are largely suppressed. These can be made visible by the use of spin-alignment echo NMR, for example. The technique is briefly described at the end of this section when SAE NMR decay curves are discussed. With SAE NMR, and with the solid echo technique as well, it is possible to overcome receiver dead-time effects and to increase the intensity of electric quadrupolar contributions to the overall NMR spectrum. A typical one is shown in Fig. 3 for x = 0.74, i.e., the orthorhombic form. Besides the central transition, a broad quadrupole powder pattern is visible which can be approximated with a Gaussian line. The full width at half maximum is approximately 62 kHz. Assuming an axially oriented electric field gradient, this corresponds to a coupling constant of 124 kHz. For comparison, this value is smaller than that roughly calculated and probed for microcrystalline lithium titanate (160 kHz) by Wagemaker et al.17 For x = 0.09 a coupling constant of 140 kHz can be estimated in the rigid lattice regime of mesoporous LixTiO2. | ||
| Fig. 3 7Li spin-alignment echo NMR spectrum recorded at 155 MHz (top). The spectrum can be deconvoluted into a broad Gaussian-shaped quadrupolar part with a full width of half maximum of ca. 62 kHz. The dashed line represents the central transition, see also Fig. 2. | ||
Coming back to the NMR central lines shown in Fig. 2, it is worth mentioning that in the present case, no indications could be found which would significantly point to a so-called heterogeneous line narrowing, that is, the emergence of a pronounced two-component line shape with a broad and narrow contribution to the overall signal. Such a feature is thought to be diagnostic for a heterogeneous dynamics owing to two magnetically decoupled (and spatially separated) spin reservoirs of fast and slowly diffusing spins.44,45
In Fig. 4 the 7Li NMR line width, deduced from the central transition, is plotted vs. temperature for the three different samples studied. Starting with a rigid line width Δνrl of 5.8 kHz for the sample with x = 0.09, Δνrl increases to 6.7 kHz (x = 0.35) until, for the sample with x = 0.74, a significantly broadened line characterized by Δνrl = 7.9 kHz is detected (see also Fig. 2). Generally, the rigid-lattice line width is directly proportional to the intensity of dipolar homonuclear Li–Li interactions; those scale with the mean interatomic Li–Li distance r which, for simple geometries, can be calculated via van Vleck's formula.43 An estimation of large x values, taking into account Li–Li coupling only, leads to approximately 8 kHz.17 Note that this estimation disregards the coupling of Li spins with strictly paramagnetic Ti3+ centers expected to be generated during Li insertion.
The beginning of motional narrowing is expected when the mean correlation time, that is approximately the residence time between two successful Li jumps, reaches the order of the inverse rigid lattice line width Δνrl. For example, at x = 0.09 line narrowing starts well below room temperature. The corresponding jump rate is then expected to range from 103 to 104 jumps per second. Most interestingly, although x has been increased from 0.09 to 0.74, that is, by a factor of eight, no drastic shift of the onset of motional narrowing towards higher temperatures is observed. At first glance, one would expect that the Δνrl becomes larger and the more the lithium ions occupy sites within the TiO2 matrix, the more the Li diffusion is slowed down. In the present case, however, something seems to (over-)compensate for the decrease expected. To our opinion, the reason should be looked for in the anatase-to-orthorhombic phase transition the TiO2 anode material undergoes during Li uptake. Recently, this phase transition has been studied by ex situ X-ray powder diffraction and in situ Raman microscopy.14 It has also been reported by 7Li magic angle spinning (MAS) NMR spectroscopy.17,18
By analyzing 7Li NMR spin–spin relaxation rates of a μm-sized powder sample showing a two-phase morphology Wagemaker et al. deduced that Li diffusivity in orthorhombic lithium titanate is enhanced compared to ion mobility in anatase. The NMR signal of Li in microcrystalline anatase (Li0.12TiO2) is reported to be 1.35 kHz in the rigid lattice while that of Li in lithium titanate is given by 11.8 kHz. Considering the shape of the rigid-lattice NMR spectrum of mesoporous Li0.09TiO2 shown in Fig. 2, which tends to be more peaked even at lower T compared to the situation in Li0.74TiO2, one might speculate that whether this sample is already a mixture of anatase and orthorhombic TiO2. While X-ray diffraction, which is being more sensitive to long-range order, does not reveal a distinct sign of a second phase in mesoporous LixTiO2 until the composition of x = 0.25.14 Raman microscopy, instead, points to the formation of nuclei of the orthorhombic phase already at x = 0.05.14 However, as powder X-ray diffraction has shown,14 up to x = 0.1 Li intercalation into the tetragonal phase continues. Regarding the spectra shown in Fig. 2, in the case of nanostructured TiO2 a deconvolution is hardly possible. Since Li ions are also expected to reside in the interfacial regions, the spectra may also be understood as a complex distribution of NMR intensities rather than a scenario of only two structurally distinct phases. Moreover, even for Li ions diffusing in a structurally well-defined single phase, a complex, heterogeneous motional narrowing may be found. Therefore, the static (ex situ) NMR measurements presented here reflect the overall Li diffusivity in LixTiO2. Interestingly, even preliminary 6Li MAS NMR measurements recorded at spinning frequencies of up to 30 kHz did not allow a reliable deconvolution of the NMR signals. Further high-resolution NMR measurements are currently underway in our lab.
To prove the above-mentioned assumption of an increase of ion diffusivity in Li-rich LixTiO2 (with x = 0.35 and 0.74) against Lix=0.09TiO2 the application of diffusion-induced SLR NMR46–48 and SAE NMR25,28,30 as well is helpful to quantify Li dynamics in terms of decay rates and microscopic activation energies. As a preliminary point, it is important to note that 7Li NMR relaxation transients, especially those recorded in the rotating frame of reference, may show a strongly non-exponential decay behavior even in those cases where a single spin reservoir is present. This is due to the spin-3/2 nature of 7Li which is exposed to both dipolar and quadrupolar interactions. From the outset, this hinders a separation of the transients into, e.g., two sub-transients. Additionally, in our case spin-relaxation phenomena taking place in the bulk may largely be different from those in the relatively large volume fraction of interfacial regions. Therefore, we analyzed the underlying magnetization transients in terms of stretched exponentials (as illustrated below) yielding a single relaxation rate which reflects the overall NMR spin relaxation.
In the lower part of Fig. 5 the temperature dependence of the 7Li NMR SLR rates measured is illustrated. Below 300 K the rates are governed by non-diffusive background relaxation due to coupling of the Li spins with the Ti3+ centers, lattice vibrations and/or other paramagnetic impurities. Expectedly, the absolute value of the background rates increases with increasing x pointing, e.g., to a larger number of Ti3+centers generated or to an increased coupling of Li spins with electrons in the conduction band, see the rates measured at a Larmor frequency of 155 MHz (Fig. 5). Sometimes these interactions can be extremely large hindering the detection of diffusion-induced contributions. In such cases other NMR techniques, as for instance mixing-time dependent SAE NMR (see below), can alternatively be used to study Li dynamics in solids.
 | ||
| Fig. 5 Arrhenius plot of the 7Li NMR SLR rates measured in the laboratory and rotating frame of reference. If not stated otherwise, data were recorded at 78 MHz. The rates 1/T1 and 1/T1ρ are shown as a function of inverse temperature 1/T. Note that, for a better comparison, the rates 1/T1 of Li0.09TiO2 are multiplied with a factor of 4. As in the case of Li0.74TiO2 the diffusion-induced rates on the low-T flank reveal a relatively weak frequency (sub-linear) dependence and show non-BPP behaviour for which 1/T1 ∝ ω−2 is expected.49 | ||
Fortunately, above room temperature the 1/T1 NMR rates shown in Fig. 5 are increasingly influenced by diffusive Li+ hopping. This is in contrast to the study reported by Wagemaker et al., who did not observe a dependence of 1/T1 on temperature for μm-sized crystallites up to ca. 500 K.17 Here, the rates increase with temperature and follow the low-T flank of a diffusion-induced NMR relaxation rate peak. At even higher temperatures the rates are expected to pass through the maximum on a log(1/T1) vs. 1/T plot. However, to prevent grain growth and to conserve the nanostructure of the materials, we restricted our measurements to 500 K. Therefore, the analysis of SLR NMR data is limited to the low-temperature limit characterized by ω0τ ≫ 1, which means that the mean correlation rate accessible is smaller than the Larmor frequency applied. In the case of Li0.74TiO2 the rates recorded at 78 MHz and at the highest temperature already indicate the appearance of a relatively broad 1/T1 peak. Note that at the rate maximum of such a peak the correlation time is expected to be in the order of the inverse Larmor frequency, i.e., the relation ω0τ ≈ 1 holds. This corresponds to Li jump rates with values in the MHz range. In the present case this is expected to be fulfilled at ca. 500 K.
The solid lines in the lower part of Fig. 5 represent fits with an Arrhenius law. The fits include only those data points recorded well above 330 K. From the slope the activation energy for short-range Li hopping can be deduced. As in the case of line narrowing, very similar activation energies are found. Interestingly, for x = 0.74 the value of Ea = 0.32(2) eV is somewhat smaller (0.37(2) eV) than that found for the sample with x = 0.09. This underpins the results from motional narrowing presented above. It is worth noting that correction procedures, taking into account the non-diffusive background rates, do not change those results much since the background rates turn out to be almost independent of temperature below T = 220 K (see also Fig. 5). Let us note that the activation energies probed here are much larger than those reported by Wagemaker et al. (0.2 eV and 0.09 eV)17 for Li diffusion in anatase and lithium titanate on the basis of spin–spin-relaxation NMR measurements.
In a more striking way the differences between the three samples probed here are illustrated by the rotating-frame SLR NMR rates of Li0.09TiO2 and Li0.74TiO2, which are exemplarily shown in the upper part of Fig. 5. The underlying magnetization transients Mρ are shown in Fig. 6; their analysis with stretched exponentials (see above) leads to the rates plotted in Fig. 5. Starting with a weaker-than-activated background relaxation, the NMR relaxation rates of Li0.74TiO2 pass into the low-T flank at a slightly lower temperature than it is the case for the sample with x = 0.09. This also holds when the background rates were approximated with a power-law function, extrapolated to higher T and subtracted from the overall rates measured. At high temperatures the rates measured at a locking frequency of 14 kHz do not follow a simply shaped rate peak. Instead, they merge into a relatively broad plateau which is an indication of complex, non-BPP49 lithium ion dynamics presumably additionally owing to the interplay with binders and carbon black present. Irrespective of that, the deviation of the SLR rates from the Arrhenius line drawn, which takes place at 420 K, points to correlation rates ranging from 105 to 106 jumps per second.
Lastly, the activation energy obtained from rotating-frame NMR data (ca. 0.53 eV (Fig. 4), note that a similar value is found for the sample with x = 0.09) is clearly larger than that deduced from NMR measurements performed in the laboratory frame of reference. In contrast to SLR NMR in the lab frame, being sensitive to jump processes in the MHz range, data recorded with the spin-locking technique are able to probe ion motions on a longer length and time scale being defined by locking frequencies ω1/2π in the kHz range. Additionally, the rather large activation energy probed is comparable with those usually found for long-range ion transport probed via dc conductivity. Thus, the SLRρ NMR rates on the low-T flank seem to be less influenced by correlation effects50–52 usually affecting SLRL NMR rates in this limit characterized by ω0τ ≫ 1. Such effects may arise from repulsive Coulomb interactions and irregular formed energy landscapes the ions are subjected to, i.e., structural disorder.52 They are known to reduce the slope on the low-T flank of SLR NMR rate peaks.
Since the maxima of the SLRρ NMR rate peaks could not be resolved, we used Jeener–Broekaert40 echoes to study spin-alignment echo (SAE) decay rates by recording single-spin motional correlation functions. Such measurements turned out to be useful to confirm the long-range nature of the activation energies probed via SLRρ NMR. Echo damping was recorded by using a three-pulse sequence; echo amplitudes were measured as a function of mixing time tm but fixed preparation time of 10 μs. The principle of SAE NMR is very similar to that of exchange NMR.25,30,53 The intensity of the echo generated after the first two pulses decreases if the jumping ions visit sites characterized by different electric field gradients (EFGs); other effects caused by (quadrupolar) spin–lattice relaxation or spin-diffusion may also contribute to the damping. A non-vanishing EFG is produced by the electric charge distribution in the direct neighborhood of the nucleus under investigation.43 The interaction of the quadrupole moment of the nucleus with an EFG alters the Zeeman levels by a certain amount of energy which is determined by the quadrupole frequency ωq. Thus, in the ideal case SAE NMR is directly sensitive to temporal changes of the site-specific quadrupole frequencies ωq,i (i = 1…n) the ions sense during hopping. Then, the decay curve represents a correlation function reflecting the probability to find an ion initially marked by ωq,1 at a site with the same ωq,1 at a later time.
In Fig. 7 typical two-time 7Li SAE NMR decay curves are shown for the samples with x = 0.09 and x = 0.74. Such curves are obtained when the intensity of the echo S2 is plotted vs. tm using a logarithmic abscissa. At very low temperatures the curves depend only weakly on temperature; presumably, in this T range echo damping is caused by spin-diffusion rather than by translational diffusion.54 Stretched exponentials with a stretching factor γ ranging from 0.32 to 0.49 are best suited to describe the dependency on mixing time tm in this non-diffusive temperature regime. In general, stretching factors deviating from γ = 1 reveal a non-Debye motional process. For example, such deviations can arise from motions in confined dimensions55 leading to a motional correlation function whose decay slows down with increasing time.
It is common to all samples that with increasing T the inflexion point of the echo decay curves shifts towards shorter tm. Concomitantly, γ steadily decreases until values of, for example, 0.32 (x = 0.09) are reached. Moreover, at a sufficiently long mixing time the curves S2(tp = const., tm) reach S2,∞ = 0 which either indicates a rather large number of quadrupole frequencies involved or which points to the influence of dipolarly coupled spins, see ref. 41 for details of spin-alignment final state amplitudes S2,∞.
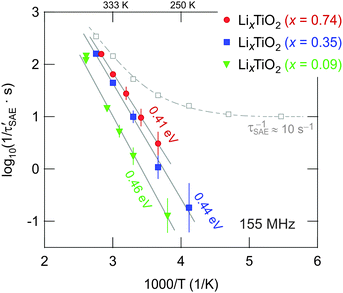
The decay rates 1/τSAE of the fitting functions, which were used to describe the two-time correlation functions S2(tp = const., tm), are exemplarily shown for x = 0.35 in Fig. 8 (grey squares). At temperatures below 200 K the rate 1/τSAE amounts to about 10 s−1 and can be identified with 1/τSAE,SD which is primarily influenced by spin diffusion (SD);20,54 the dashed line in Fig. 8 is to guide the eye. Above 250 K the SAE NMR rates increase due to translational Li hopping. To correct the rates 1/τSAE for any non-diffusive background contribution, we calculated the difference 1/τ′SAE = 1/τSAE – 1/τSAE,SD. The resulting rates 1/τ′SAE (x = 0.35) follow an Arrhenius law with an activation energy of Ea,SAE = 0.44(2) eV (see Fig. 8). The same procedure is applied to those data obtained for x = 0.09 and x = 0.74. Starting from x = 0.09, the activation energy decreases from 0.46 eV to 0.41 eV (see Fig. 8). This is consistent with the fact that the lowest rates were obtained for the sample with x = 0.09. Activation energies from SAE NMR are somewhat lower than those determined from SLR NMR in the rotating frame of reference, while the lowest values were found by SLR NMR performed in the laboratory frame of reference. These differences illustrate that there are different types of hopping motion that each experiment type is sensitive to. Usually, the low-T flank of SLR NMR in the lab frame is sensitive to short-range Li dynamics rather than to long-range ion motion. The latter seems to be better probed by rotating frame methods carried out at much lower (effective) resonance frequencies (14 kHz, see above).
Although the differences in 1/τ′SAE (and Ea,SAE) found are small for the three samples investigated, they particularly underpin the trend obtained from SLRρ NMR. Thus, with the increase of x, which initiates the above mentioned phase transformation, Li diffusion is slightly increased rather than decreased. Besides other advantages of the mesoporous anode material this effect is highly desirable because it is expected to contribute to the excellent rate performance documented.14
4 Summary and outlook
The present study represents one of the very first examples that takes advantage of solid-state NMR to keep track of Li dynamics during charging a (mixed conducting) TiO2-based anode in a lithium-ion battery. The diffusion parameters extracted from the various NMR methods applied point to Li dynamics being almost independent of the amount of Li inserted into the transition metal oxide. In fact, Li bulk diffusivity, which might also be influenced by surface diffusivity, seems to increase rather than to decrease with increasing x in LixTiO2. This behavior might directly be related to the phase transformation which takes place during charging. The anode material, which initially crystallizes mainly with the anatase structure, gradually transforms into orthorhombic LixTiO2 upon Li insertion.In particular, results from SAE NMR, being sensitive to relatively slow but long-range, translational ion dynamics underline this behavior. The corresponding activation energies obtained from relaxation NMR in the rotating frame of reference and SAE NMR, respectively, range from 0.41 eV to 0.53 eV. Such relatively large values are expected for Li+ ion transport proceeding on a longer length scale within the TiO2 matrix. Besides NMR and a few other techniques, in the case of lithium such values are otherwise only obtainable by dc conductivity measurements. However, when dealing with mixed conductors, impedance spectroscopy needs special setups and careful sample preparation to separate ionic from electronic contributions. NMR, however, is a contactless method with no requirement for special sample (post-)preparation.
Finally, both the stretching of the two-time SAE NMR correlation functions and the lower activation energy found by SLR NMR performed in the laboratory frame of reference point to a complex Li dynamics present. This manifests in activation energies which depend on the time scale the method applied is sensitive to. Further experiments, especially those using 6Li (SAE) NMR techniques might be useful to shed light on the deviation from simple Debye behavior observed.
Further notes
The authors declare no competing financial interest.Acknowledgements
Financial support from the Deutsche Forschungsgemeinschaft (DFG FOR 1277, project 7 “Li diffusion in nanostructured materials”) is highly appreciated. M.W. thanks the Leibniz University Hannover for further financial support (grant “Wege in die Forschung II”). P.G. Bruce is grateful to the EPSRC including SUPERGEN and the program grant “Nanoionics” for financial support.References
- P. G. Bruce, B. Scrosati and J.-M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930 CrossRef CAS PubMed
.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed
.
- M. S. Whittingham, Chem. Rev., 2004, 104, 4271 CrossRef CAS
.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587 CrossRef CAS
.
- M. V. Reddy, G. V Subba Rao and B. V. R. Chowdari, Chem. Rev., 2013, 113, 5364 CrossRef CAS PubMed
.
- R. Marom, S. F. Amalraj, N. Leifer, D. Jacob and D. Aurbach, J. Mater. Chem., 2011, 21, 9938 RSC
.
- J. Maier, Nat. Mater., 2005, 4, 805 CrossRef CAS
.
- M. Wagemaker and F. M. Mulder, Acc. Chem. Res., 2012, 46, 1206 CrossRef PubMed
.
- Y. Wang and G. Cao, Adv. Mater., 2008, 20, 2251 CrossRef CAS
.
- D. Deng, M. G. Kim, J. Y. Lee and J. Cho, Energy Environ. Sci., 2009, 2, 818 CAS
.
- H.-K. Song, K. Lee, M. G. Kim, L. F. Nazar and J. Cho, Adv. Funct. Mater., 2010, 20, 3818 CrossRef CAS
.
- A. Vu, Y. Qian and A. Stein, Adv. Energy Mater., 2012, 2, 1056 CrossRef CAS
.
- Y. Ren, Z. Ma and P. G. Bruce, Chem. Soc. Rev., 2012, 41, 4909 RSC
.
- Y. Ren, L. J. Hardwick and P. G. Bruce, Angew. Chem., Int. Ed., 2010, 49, 2570 CrossRef CAS PubMed
.
- M. Zukalová, M. Kalbáč, L. Kavan, I. Exnar and M. Graetzel, Chem. Mater., 2005, 17, 1248 CrossRef
.
- G. Sudan, E. Baudrin, D. Larcher and J.-M. Tarascon, J. Mater. Chem., 2005, 15, 1263 Search PubMed
.
- M. Wagemaker, R. van de Krol, A. P. M. Ken gens, A. A. van Well and F. M. Mulder, J. Am. Chem. Soc., 2001, 123, 11454 CrossRef CAS PubMed
.
- V. Luca, L. Hanley, N. K. Robers and R. F. Howe, Chem. Mater., 1999, 11, 2089 CrossRef CAS
.
- M. Vijayakumar, S. Kerisi, C. Wang, Z. Nie, K. M. Rosso, Z. Yang, G. Graff, J. Liu and J. Hu, J. Phys. Chem. C, 2009, 113, 14567 CAS
.
- M. Wilkening, C. Lyness, A. R. Armstrong and P. G. Bruce, J. Phys. Chem. C, 2009, 113, 4741 CAS
.
- M. Wilkening, R. Amade, W. Iwaniak and P. Heitjans, Phys. Chem. Chem. Phys., 2007, 9, 1239 RSC
.
- M. Wagemaker, A. P. M. Kentgens and F. M. Mulder, Nature, 2002, 418, 397 CrossRef CAS PubMed
.
- M. Wagemaker, W. J. H. Borghols, E. R. H. van Eck, A. P. M. Kentgens, G. J. Kearley and F. M. Mulder, Chem.–Eur. J., 2007, 13, 2023 CrossRef CAS PubMed
.
- M. Wilkening, W. Küchler and P. Heitjans, Phys. Rev. Lett., 2006, 97, 065901 CrossRef CAS
.
- M. Wilkening and P. Heitjans, ChemPhysChem, 2012, 13, 53 CrossRef CAS PubMed
.
- M. Wilkening, A. Kuhn and P. Heitjans, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 054303 CrossRef
.
- F. Qi, G. Hinze, R. Böhmer, H. Sillescu and H. Zimmermann, Chem. Phys. Lett., 2000, 328, 257 CrossRef CAS
.
- R. Böhmer and F. Qi, Solid State Nucl. Magn. Reson., 2007, 31, 28 CrossRef PubMed
.
- M. Wilkening and P. Heitjans, J. Phys.: Condens. Matter, 2006, 18, 9849 CrossRef CAS
.
- R. Böhmer, K. Jeffrey and M. Vogel, Prog. Nucl. Magn. Reson. Spectrosc., 2007, 50, 87 CrossRef PubMed
.
- M. Wilkening, W. Iwaniak, J. Heine, V. Epp, A. Kleiner, M. Behrens, G. Nuspl, W. Bensch and P. Heitjans, Phys. Chem. Chem. Phys., 2007, 9, 6199 RSC
.
- V. Epp, S. Nakhal, M. Lerch and M. Wilkening, J. Phys.: Condens. Matter, 2013, 25, 195402 CrossRef CAS PubMed
.
- A. Van der Ven, J. Bhattacharya and A. A. Belak, Acc. Chem. Res., 2013, 46, 1216 CrossRef CAS PubMed
.
- M. Wilkening, J. Heine, C. Lyness, A. Armstrong and P. Bruce, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 064302 CrossRef
.
-
E. Fukushima and S. B. W. Roeder, Experimental Pulse NMR: A Nuts and Bolts Approach, New Ed., Westview Press, Boulder, 1993 Search PubMed
.
- D. Ailion and C. Slichter, Phys. Rev. Lett., 1964, 12, 168 CrossRef CAS
.
- C. Slichter and D. Ailion, Phys. Rev. A: At., Mol., Opt. Phys., 1964, 135, 1099 CrossRef
.
- D. Ailion and C. Slichter, Phys. Rev. A: At., Mol., Opt. Phys., 1965, 137, 235 CrossRef
.
- R. Böhmer, J. Magn. Reson., 2000, 147, 78 CrossRef PubMed
.
- J. Jeener and P. Broekaert, Phys. Rev., 1967, 157, 232 CrossRef CAS
.
- F. Qi, G. Diezemann, H. Böhm, J. Lamber and R. Böhmer, J. Magn. Reson., 2004, 169, 225 CrossRef CAS PubMed
.
- M. Wilkening, V. Epp, A. Feldhoff and P. Heitjans, J. Phys. Chem. C, 2008, 112, 9291 CAS
.
-
A. Abragam, The Principles of Nuclear Magnetism, Clarendon, Oxford, 1961 Search PubMed
.
- S. Indris and P. Heitjans, J. Non-Cryst. Solids, 2002, 307–310, 555 CrossRef CAS
.
- M. Wilkening, S. Indris and P. Heitjans, Phys. Chem. Chem. Phys., 2003, 5, 2225 RSC
.
- H. Buschmann, J. Dölle, S. Berendts, A. Kuhn, P. Bottke, M. Wilkening, P. Heitjans, A. Senyshyn, H. Ehrenberg, A. Lotnyk, V. Duppel, L. Kienle and J. Janek, Phys. Chem. Chem. Phys., 2011, 13, 19378 RSC
.
- A. Kuhn, M. Kunze, P. Sreeraj, H. D. Wiemhöfer, V. Thangadurai, M. Wilkening and P. Heitjans, Solid State Nucl. Magn. Reson., 2012, 42, 2 CrossRef CAS PubMed
.
- A. Kuhn, P. Sreeraj, R. Pöttgen, H.-D. Wiemhöfer, M. Wilkening and P. Heitjans, J. Am. Chem. Soc., 2011, 133, 11018 CrossRef CAS PubMed
.
- N. Bloembergen, E. M. Purcell and R. V. Pound, Phys. Rev., 1948, 73, 679 CrossRef CAS
.
- K. Funke, Prog. Solid State Chem., 1993, 22, 111 CrossRef CAS
.
- M. Meyer, P. Maass and A. Bunde, Phys. Rev. Lett., 1993, 71, 573 CrossRef CAS
.
-
A. Bunde, W. Dieterich, P. Maass and M. Meyer, in Diffus. Condens. Matter – Methods, Mater. Model, ed. P. Heitjans and J. Kärger, Springer, Berlin, 2005, p. 813 Search PubMed
.
- B. Koch and M. Vogel, Solid State Nucl. Magn. Reson., 2008, 34, 37 CrossRef CAS PubMed
.
- C. Brinkmann, S. Faske, B. Koch and M. Vogel, Z. Phys. Chem., 2010, 224, 1535 CrossRef CAS
.
- M. Wilkening and P. Heitjans, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 24311 CrossRef
.
| This journal is © the Owner Societies 2014 |