 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Kinetics of CH2OO reactions with SO2, NO2, NO, H2O and CH3CHO as a function of pressure†
Daniel
Stone
a,
Mark
Blitz
*ab,
Laura
Daubney
a,
Neil U. M.
Howes
a and
Paul
Seakins
ab
aSchool of Chemistry, University of Leeds, Leeds, UK. E-mail: m.blitz@leeds.ac.uk
bNational Centre for Atmospheric Science, University of Leeds, Leeds, UK
First published on 20th November 2013
Abstract
Kinetics of CH2OO Criegee intermediate reactions with SO2, NO2, NO, H2O and CH3CHO and CH2I radical reactions with NO2 are reported as a function of pressure at 295 K. Measurements were made under pseudo-first-order conditions using flash photolysis of CH2I2–O2–N2 gas mixtures in the presence of excess co-reagent combined with monitoring of HCHO reaction products by laser-induced fluorescence (LIF) spectroscopy and, for the reaction with SO2, direct detection of CH2OO by photoionisation mass spectrometry (PIMS). Rate coefficients for CH2OO + SO2 and CH2OO + NO2 are independent of pressure in the ranges studied and are (3.42 ± 0.42) × 10−11 cm3 s−1 (measured between 1.5 and 450 Torr) and (1.5 ± 0.5) × 10−12 cm3 s−1 (measured between 25 and 300 Torr), respectively. The rate coefficient for CH2OO + CH3CHO is pressure dependent, with the yield of HCHO decreasing with increasing pressure. Upper limits of 2 × 10−13 cm3 s−1 and 9 × 10−17 cm3 s−1 are placed on the rate coefficients for CH2OO + NO and CH2OO + H2O, respectively. The upper limit for the rate coefficient for CH2OO + H2O is significantly lower than has been reported previously, with consequences for modelling of atmospheric impacts of CH2OO chemistry.
1. Introduction
Criegee intermediates, carbonyl oxide biradicals with the general formula CR2OO, are principally produced in the atmosphere following ozonolysis of unsaturated volatile organic compounds (VOCs) and are key species in the tropospheric oxidation of both biogenic and anthropogenic compounds.1,2 The exothermicity of ozonolysis reactions leads to production of vibrationally excited Criegee intermediates with sufficient energy to undergo unimolecular decomposition to products including OH and HO2,3–6 representing a significant source of these key oxidising species in certain important environments.7–9 However, collisional quenching of the nascent excited Criegee intermediate by N2 or O2, to produce stabilised Criegee intermediates, is competitive with the unimolecular decomposition processes at ambient pressures,1,5 and reactions of stabilised Criegee intermediates have the potential to impact atmospheric budgets of NOx (NOx = NO + NO2), NO3, O3, HOx (HOx = OH + HO2), SO2, H2SO4, sulfate aerosol and secondary organic aerosol (SOA).5,10–17Despite their potential importance in atmospheric chemistry, and thus in the assessment and prediction of issues such as air quality and climate change, direct observations of Criegee intermediates have only recently been achieved.10–12,18–20 Kinetics and product yields of Criegee intermediate reactions currently used in atmospheric models are subject to large uncertainties, owing to the reliance of previous investigations on indirect techniques involving measurements of stable species in complex ozonolysis experiments, in which there are several potential sources and sinks of the measured species.1,2 Welz et al.10 reported the first direct measurements of Criegee intermediate kinetics, where the photolysis of CH2I2 in the presence of O2 was used to generate the CH2OO Criegee intermediate at low pressure (4 Torr) and, using synchrotron photoionisation mass spectrometry (PIMS) at the Advanced Light Source (ALS), demonstrated unequivocally that the Criegee intermediate, CH2OO, was being monitored:
| CH2I2 + hν → CH2I + I | (R1) |
| CH2I + O2 → CH2OO + I | (R2a) |
While reactions of CH2OO with NO and water vapour were reported to be slow, the reactions of CH2OO with SO2 and NO2 were shown to be significantly faster than indicated by the indirect methods. Rate coefficients for both CH2OO + SO2 and CH2OO + NO2, measured at a pressure of 4 Torr and temperature of 298 K, were both approximately 1000 times greater than previously assigned, implying a more significant role of Criegee intermediate chemistry in the atmosphere than expected.
The ability to produce CH2OO following photolysis of CH2I2 in the presence of O210 has also facilitated spectroscopic investigations of CH2OO in the infrared19 and ultraviolet,20 and has been used to demonstrate the production of NO3 in the reaction of CH2OO with NO2.21 Subsequent work at the ALS has investigated the reactions of CH2OO with acetone, acetaldehyde and hexafluoroacetone at low pressures,11 with theoretical investigation22 of the reaction between CH2OO and acetaldehyde (CH3CHO) indicating pressure dependence of the reaction and collisional stabilisation of nascent reaction adducts to produce secondary ozonides (SOZs) at higher pressures which subsequently decompose to generate organic acids.
Taatjes et al.12 have also recently demonstrated production of the CH3CHOO Criegee intermediate following photolysis of CH3CHI2 in the presence of O2. The structure of the CH3CHOO Criegee intermediate gives rise to the possibility of syn- and anti-conformers, with the conformers sufficiently different in energy, and with a barrier to conversion, leading to the potential for their behaviour as distinct species. Using the synchrotron PIMS technique, Taatjes et al.12 were not only able to identify both the syn- and anti-CH3CHOO conformers, but were also able to assign separate rate coefficients for reactions of the two conformers with SO2 and water vapour. The anti-conformer was shown to display greater reactivity towards both SO2 and H2O compared to the syn-conformer, with rate coefficients for reactions of both syn- and anti-conformers with SO2 greater than previously expected.12
Field observations in a boreal forest in Finland have provided further evidence for rapid reactions between Criegee intermediates and SO2, with measurements identifying the presence of oxidising species other than OH which are able to oxidise SO2 to SO3 and ultimately to produce H2SO4.23 The presence of the unknown oxidising species was shown to be related to emissions of biogenic alkenes, and it was postulated that Criegee intermediates may be responsible, with laboratory measurements of H2SO4 production during alkene ozonolysis reactions in the presence of SO2 and OH scavengers providing further support for the action of Criegee intermediates as atmospheric oxidants of SO2.23
Implementation of increased Criegee intermediate + SO2 reaction rates in atmospheric models has been shown to improve model simulations of H2SO4 in forested regions in Finland and Germany,14 and global modelling has shown that while global production of H2SO4 increases by only 4%, there are increases of up to 100% in the boundary layer in tropical forests.15 Further modelling work has shown that reactions of Criegee intermediates with SO2 can compete with OH + SO2 in a number of regions, and that Criegee + SO2 reactions may be the dominant removal mechanism for SO2 in certain areas and are major contributors to sulfate aerosol formation on a regional scale.17 Air quality modelling over the U.S. displayed limited impacts of increased Criegee + SO2 reaction rates on sulfate aerosol production in this region, but the impacts were shown to be highly dependent on the competition between Criegee + SO2 and Criegee + H2O, with a combination of increased Criegee + SO2 and decreased Criegee + H2O reaction rates leading to enhanced sulfate aerosol concentrations.16 However, such studies have largely been based on the low pressure data for CH2OO + SO2 reported by Welz et al.10 and there is considerable uncertainty regarding the upper limit for CH2OO + H2O.2,17
Theoretical work has provided support for rapid reactions between Criegee intermediates and SO2,13,24 with reactions proceeding via the initial barrierless formation of a cyclic secondary ozonide, and has enabled prediction of potential effects of pressure.13 For CH2OO + SO2, it has been predicted that the reaction products at atmospheric pressure will be a mixture of HCHO + SO3 (∼68%), formyl sulfinic ester (HC(O)OS(O)OH) (∼15%) and a singlet bisoxy diradical (CH2(O)O) + SO2 (∼17%).13 In contrast, reactions of larger Criegee intermediates, including CH3CHOO, at ambient pressures are expected to result in production of stabilised secondary ozonide species, with little formation of SO3, and therefore little impact on H2SO4 and sulfate aerosol.13 Investigation of the reaction products and pressure dependence of Criegee intermediate reactions is thus essential to the accurate determination of their atmospheric impacts.
The yield of CH2OO Criegee intermediates following CH2I2 photolysis in O2 was studied by Huang et al.,25 and in our previous work,26 as a function of pressure. Both investigations indicate that the initial reaction between CH2I radicals and O2(R2) produces a chemically activated species, CH2IO2#, which decomposes at low pressures to produce CH2OO + I (R2a), but is collisionally stabilised at higher pressures to produce the CH2IO2 peroxy radical (R2b).
| CH2I2 + hν → CH2I + I | (R1) |
| CH2I + O2 → CH2IO2# | (R2) |
| CH2IO2# → CH2OO + I | (R2a) |
| CH2IO2# + M → CH2IO2 + M | (R2b) |
Our previous work26 indicates a yield of ∼18% CH2OO following photolysis of CH2I2 in air at 760 Torr, with recent results from Huang et al.27 in reasonable agreement. This result has potential significance for modelling of atmospheric chemistry in iodine-rich regions,28–31 and also indicates potential for pressure dependent studies of CH2OO kinetics using photolysis of CH2I2 in O2.
In this work, we report kinetics of CH2OO reactions with SO2, NO2, NO, H2O and CH3CHO at pressures between 25 and 450 Torr at a temperature of 295 K, using photolysis of CH2I2–O2–N2 mixtures under pseudo-first-order conditions combined with monitoring of the HCHO reaction products by laser-induced fluorescence (LIF) spectroscopy, and, for the CH2OO + SO2 reaction at ∼1.5 Torr, direct monitoring of CH2OO by photoionisation mass spectrometry (PIMS). We also report kinetics of the CH2I + NO2 reaction at pressures between 25 and 300 Torr at 295 K.
2. Experimental
2.1 Laser-induced fluorescence experiments
Apparatus and experimental procedures for the laser-induced fluorescence (LIF) experiments have been described elsewhere in detail,26,32 therefore only a brief description is given here. Kinetics of CH2OO reactions were studied by monitoring of HCHO reaction products by LIF spectroscopy. Radicals were generated by the laser flash photolysis of CH2I2–O2–N2 gas mixtures (R1 and R2) with the addition of excess co-reagent (NO2, NO, SO2, H2O or CH3CHO) to ensure pseudo-first-order conditions. Experiments to investigate CH2I + NO2 kinetics were performed in the absence of O2, while those to investigate CH2OO + NO2 were performed using a limited range of NO2 concentrations in order to avoid production of HCHO through the reaction of CH2I with NO2 (see Section 3.1), whilst maintaining pseudo-first-order conditions.CH2I2 (Sigma-Aldrich, 99%) was used as a dilute gas in N2 either by filling a glass bulb containing liquid CH2I2 with N2 or by bubbling a slow flow of N2 through liquid CH2I2. Reagents (NO, NO2, SO2, CH3CHO) were prepared at known concentrations in N2 and stored in glass bulbs. NO (BOC Special Gases, 99.5%) was purified prior to use by a series of freeze–pump–thaw cycles. CH2I2, CH3CHO (Sigma-Aldrich, 99.5%), NO2 (Sigma-Aldrich, 99.5%), SO2 (Sigma-Aldrich, 99.9%), N2 (BOC, 99.99%) and O2 (BOC, 99.999%) were used as supplied. Water vapour was added to the gas mixture by bubbling a known flow of N2 gas through a bubbler containing deionised water at a known temperature. Gases were mixed in a gas manifold and passed into a six-way cross reaction cell at known flow rates (determined by calibrated mass flow controllers). The pressure in the reaction cell was monitored by a capacitance manometer (MKS Instruments, 626A) and controlled by throttling the exit valve to the reaction cell. The total gas flow rate through the reaction cell was adjusted with total pressure to maintain an approximately constant gas residence time in the cell (∼0.1 s). All experiments were performed at T = (295 ± 2) K unless stated otherwise.
For experiments using NO2, NO, CH3CHO or H2O as co-reagents, initiation of chemistry within the cell was achieved using an excimer laser (KrF, Tui ExciStar M) operating at λ = 248 nm with typical laser fluence in the range 30–80 mJ cm−2. Experiments in which SO2 was present as the co-reagent were performed at a photolysis wavelength of 355 nm (typical fluence ∼ 150 mJ cm−2), generated by frequency tripling the output of a Nd:YAG laser (Spectron Laser Systems) to avoid potential multi-photon photolysis of SO2 at shorter wavelengths.33–35
Production of HCHO was monitored by laser-induced fluorescence (LIF) of HCHO at λ ∼ 353.1 nm.36 Approximately 2 to 4 mJ pulse−1 of laser light at ∼353.1 nm was generated by a dye laser (Lambda Physik, FL3002) operating on DMQ/dioxirane dye and pumped by a 308 nm excimer laser generating ∼50 mJ pulse−1 (XeCl, Lambda Physik LPX100). The output of the dye laser was passed through the reaction cell in an orthogonal axis to the 248 nm/355 nm photolysis laser output, with HCHO fluorescence detected in the visible region of the spectrum by a channel photomultiplier (CPM, Perkin-Elmer C1943P) orthogonal to both the photolysis laser and the LIF excitation laser beams. A Perspex filter was used to prevent scattered laser light from the photolysis laser and the LIF excitation laser reaching the CPM. The HCHO fluorescence signal was monitored as a function of time following photolysis of CH2I2 by varying the time delay between firing the photolysis laser and the LIF excitation laser through use of a delay generator (SRS DG535). Results from between 5 and 20 photolysis shots were typically averaged prior to analysis.
2.2 Photoionisation mass spectrometry experiments
Photoionisation mass spectrometry (PIMS) experiments were performed in this work to determine the kinetics of CH2OO + SO2 at low pressure (∼1.5 Torr) and 295 K by direct monitoring of CH2OO in reactions performed under pseudo-first-order conditions. The PIMS apparatus has been described previously in detail32,37,38 and only a brief description is given here. Gas mixtures of CH2I2–O2–N2 and CH2I2–O2–N2–SO2 were prepared in a gas handling line, with reagents and reagent preparation as described above for the LIF experiments, and introduced to the steel reaction flow tube (10.5 mm internal diameter, 70 cm in length) via calibrated mass flow controllers. The pressure in the reaction flow tube was monitored by a capacitance manometer (MKS Instruments, 626A) and controlled by throttling the exit valve to the flow tube.Chemistry was initiated by a pulsed excimer laser (Lambda Physik, Compex 205) at a wavelength of 248 nm, with typical fluence of ∼50 mJ cm−2, through reactions (R1) and (R2). A representative sample from the reaction mixture effused into a high vacuum chamber (<10−5 Torr, maintained by diffusion and turbo pumps) via a 1 mm pinhole situated in the sidewall of the reaction flow tube. Components of the gas mixture were photoionised using 118 nm vacuum ultraviolet (VUV) laser light (typically 1011 photons pulse−1), generated by frequency tripling of the third harmonic of a Nd:YAG laser (Continuum Powerlite, 8010) in a Xe gas cell, and passed across the effusing gas flow within 2–3 mm of the sampling pinhole. VUV light of 118 nm (equivalent to 10.5 eV) is sufficiently energetic to ionise CH2OO (threshold = 10.02 eV), but is below the threshold required to ionise other isomers at m/z = 46 (dioxirane, threshold = 10.82 eV; formic acid, threshold = 11.33 eV).10 Ions were sampled by the time of flight mass spectrometer (TOF-MS, Kore Technology Ltd), and detected by an electron multiplier. The ion signals were amplified and boxcar averaged on an oscilloscope and then stored on the control computer. The ion signals were monitored as a function of time following photolysis of CH2I2 by varying the time delay between the excimer laser and the Nd:YAG laser, used to generate the VUV radiation, through use of a delay generator (SRS DG35). These kinetic traces consisted of typically 200 time points, with typically between 10 and 25 shot averaging per time point.
3. Results and discussion
3.1 Photolysis of CH2I2–O2–N2 mixtures
Fig. 1 shows the HCHO fluorescence signal following photolysis of CH2I2–O2–N2 mixtures (i.e. in the absence of any additional co-reagent), resulting in production of HCHO through reactions (R1)–(R6):26,32| CH2I2 + hν → CH2I + I | (R1) |
| CH2I + O2 → CH2OO + I | (R2a) |
| CH2I + O2 + M → CH2IO2 + M | (R2b) |
| CH2OO + I → HCHO + IO | (R3) |
| CH2IO2 + I → CH2IO + IO | (R4) |
| CH2IO2 + CH2IO2 → 2CH2IO + O2 | (R5) |
| CH2IO → HCHO + I | (R6) |
Previous work in this laboratory26 has shown that the yields of CH2OO and CH2IO2 from (R2) are dependent on pressure, owing to initial formation of the excited species CH2IO2#, which can either decompose to produce the CH2OO Criegee intermediate and iodine atoms (R2a) or can be collisionally stabilised to produce the peroxy radical CH2IO2(R2b). Since subsequent reactions of both CH2OO and CH2IO2 in the absence of any additional co-reagent result in production of HCHO, there is no change in the total HCHO yield as a function of pressure following photolysis of CH2I2–O2–N2 mixtures.
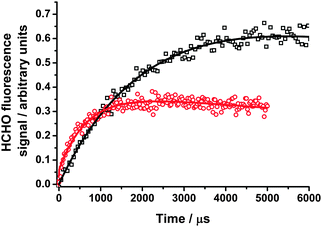 | ||
| Fig. 1 HCHO fluorescence signals at 200 Torr following photolysis of CH2I2 in the presence of O2 in the absence of any co-reagent (black open squares) and in the presence of NO2 (red open circles). The fits to eqn (1) are shown by the solid lines, and give kg′ = (460 ± 30) s−1 in the absence of any additional co-reagent and kg′ = (1490 ± 50) s−1 in the presence of NO2. The ratio of S1 (eqn (1)) in the presence of NO2 to that in the absence of NO2 is 0.37. | ||
Production of HCHO in reactions (R1)–(R6) can be approximated by eqn (1):26,32
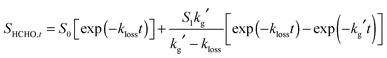 | (1) |
In the absence of any additional co-reagent, the first-order rate coefficient approximating the production of HCHO, kg′, was found to vary from ∼300 s−1 to ∼3500 s−1, depending on the concentration of CH2I2, and thus of I atoms, in the system, in keeping with the work of Welz et al.10 and Taatjes et al.11 Some initial HCHO production was observed owing to multi-photon photolysis of CH2I2 and the subsequent rapid reaction of 3CH2 with O2, with S0 typically no greater than 5–10% of S1.39–43
3.2 CH2OO + SO2
The reaction of CH2OO with SO2(R7) was investigated in separate experiments using the PIMS method to monitor CH2OO and the LIF method to monitor HCHO production.| CH2OO + SO2 → HCHO + SO3 | (R7) |
Experiments using the PIMS method were performed at a total pressure of 1.5 Torr. Fig. 2 shows a typical decay for CH2OO observed in the presence of excess SO2, with the pseudo-first-order rate coefficient for CH2OO decay found by least-squares fitting to eqn (2):
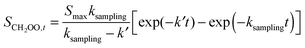 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s−1, described in detail by Baeza-Romero et al.38).
000 s−1, described in detail by Baeza-Romero et al.38).
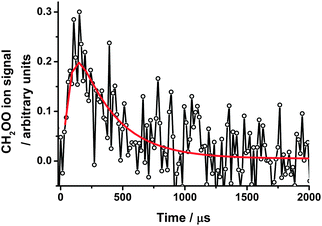 | ||
| Fig. 2 CH2OO ion signals at 1.5 Torr following photolysis of CH2I2–O2–N2 in the presence of SO2, with the fit to eqn (2) (solid red line). For these data, k′ = (3310 ± 450) s−1. | ||
The bimolecular rate coefficient for CH2OO + SO2 (k7) determined using the PIMS method at 1.5 Torr was (3.6 ± 0.5) × 10−11 cm3 s−1 (Fig. 3), similar to the value of (3.9 ± 0.7) × 10−11 cm3 s−1 at 4 Torr reported by Welz et al.10 and several orders of magnitude greater than the values typically used in atmospheric models.
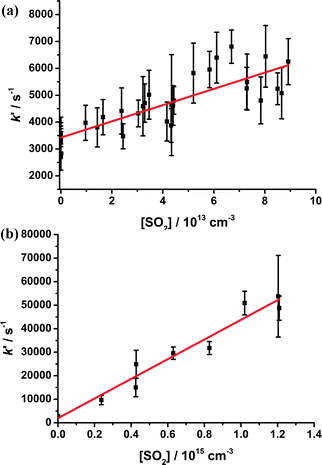 | ||
| Fig. 3 (a) Pseudo-first-order rate coefficients (k′) at 1.5 Torr, derived from fits to eqn (2), for the decay of the CH2OO ion signal (m/z = 46, ionised using VUV radiation at 118 nm) following photolysis of CH2I2–O2–N2 in the presence of SO2. Error bars are 1σ. The fit to the data (shown in red) gives the bimolecular rate coefficient for CHOO + SO2 (k7); (b) pseudo-first-order rate coefficients (kg1′) for the rapid HCHO production at 250 Torr following photolysis of CH2I2–O2–N2 in the presence of SO2 derived from fits to eqn (3). Error bars are 1σ. The fit to the data (shown in red) gives the bimolecular rate coefficient for CHOO + SO2 (k7). | ||
The LIF experiments monitoring HCHO production from CH2OO + SO2 were performed over the pressure range 50–450 Torr, with SO2 concentrations in the range 2.4 × 1014 to 1.6 × 1015 cm−3. The HCHO growth (Fig. 4) was observed to display biexponential behaviour, with no decrease in the total HCHO yield compared to experiments performed in the absence of any co-reagent, indicating complete titration of both CH2OO and CH2IO2 to HCHO. Kinetic parameters were determined by fitting to eqn (3):
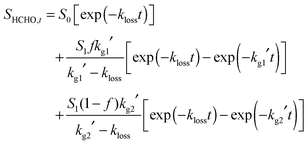 | (3) |
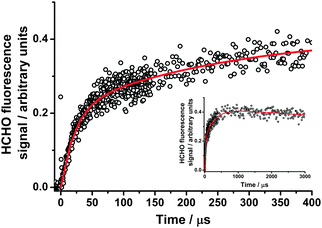 | ||
Fig. 4 HCHO fluorescence signals at 250 Torr following photolysis of CH2I2–O2–N2 in the presence of SO2, with the fit to eqn (3) (solid red lines). The inset panel shows the evolution of the signal to longer times. For these data, kg1′ = (45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ± 2240) s−1; kg2′ = (3580 ± 280) s−1; kloss = (40 ± 9) s−1; f = (0.49 ± 0.01); S1 = (0.43 ± 0.01). 500 ± 2240) s−1; kg2′ = (3580 ± 280) s−1; kloss = (40 ± 9) s−1; f = (0.49 ± 0.01); S1 = (0.43 ± 0.01). | ||
The initial fast growth of HCHO displayed a linear dependence on [SO2], while the slower growth was independent of [SO2] and at a similar rate to the observed HCHO production in the absence of any additional co-reagent. The yields of HCHO from the faster growth process were consistent with production from CH2OO + SO2, while those from the slower process were consistent with production from reactions of CH2IO2 (i.e.reactions (R4)–(R6)). We thus determine k7 from linear fits of kg1′ (eqn (3)) against [SO2]. The validity of describing the system using eqn (3) is discussed in our previous work.26
Fig. 5 and Table 1 show the values of k7 as a function of pressure. No significant dependence of k7 on pressure was observed, with an average value of (3.42 ± 0.42) × 10−11 cm3 s−1 for all experiments (PIMS and LIF) described in this work (all errors are 1σ unless stated otherwise). Moreover, there is no significant change in the HCHO yield from the reaction of CH2OO with SO2 as a function of pressure, indicating there is little stabilisation of reaction products. These results are consistent with the low pressure results obtained by Welz et al.10 and theoretical work by Vereecken et al.,13 and support arguments for an increased role of CH2OO + SO2 in the atmosphere. Taatjes et al.12 have also shown that the reaction of the C2 Criegee intermediate, CH3CHOO, with SO2 at a pressure of 4 Torr is also significantly faster than previously expected, potentially indicating an increased role for CH3CHOO + SO2 in the atmosphere. However, theoretical calculations predict that reactions of larger Criegee intermediates will exhibit pressure dependence,13 and that production of SO3 in reactions of larger Criegee intermediates at atmospheric pressures is unlikely owing to stabilisation of SO2–Criegee intermediate complexes to produce secondary ozonide species, thus reducing the impacts of SO2 + Criegee intermediate reactions on H2SO4 and sulfate aerosol production.13 Field observations and laboratory studies by Mauldin et al.23 indicate that larger Criegee intermediates, such as those produced in the ozonolysis of monoterpenes, do impact on atmospheric concentrations of H2SO4 through oxidation of SO2, but that the impacts may not be as great as those reported for CH2OO, potentially owing to stabilisation of reaction products. Further work is thus required to investigate the effects of pressure on the reactions of larger Criegee intermediates. Moreover, modelled impacts of increases in the rates of Criegee intermediate reactions with SO2 are highly dependent on the competition with rates of Criegee intermediate reactions with water vapour. We thus investigate CH2OO + H2O in Section 3.6.
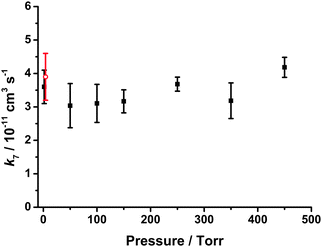 | ||
| Fig. 5 Bimolecular rate coefficients for CH2OO + SO2 (k7) as a function of pressure. Error bars are 1σ. The plot includes results from the PIMS experiments (at 1.5 Torr) and the LIF experiments (pressures ≥ 50 Torr). The data point shown by the red open circle is that determined by Welz et al.10 | ||
3.3 CH2I + NO2
Production of HCHO following photolysis of CH2I2–NO2–N2 mixtures was examined as a function of pressure to facilitate assessment of the competition between CH2I + O2(R2) and CH2I + NO2(R8) in CH2OO + NO2 experiments (Section 3.4).| CH2I + NO2 → HCHO + products | (R8) |
The production of HCHO could be described by eqn (1) (above), where kg′ = k8[NO2], with concentrations of NO2 between 1 × 1014 and 9 × 1014 cm−3. Pseudo-first-order rate coefficients (kg′) were in the range ∼5000 to 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s−1, and typically large compared to the rate coefficients describing HCHO production in the absence of any additional co-reagent (Section 3.1). The bimolecular rate coefficient k8 was determined from plots of kg′ against [NO2] at each pressure (Fig. S1, ESI†), and was found to increase with increasing pressure (Fig. S2 and Table S1, ESI†), with a corresponding decrease in the HCHO yield as the pressure was increased (Fig. S3, ESI†).
000 s−1, and typically large compared to the rate coefficients describing HCHO production in the absence of any additional co-reagent (Section 3.1). The bimolecular rate coefficient k8 was determined from plots of kg′ against [NO2] at each pressure (Fig. S1, ESI†), and was found to increase with increasing pressure (Fig. S2 and Table S1, ESI†), with a corresponding decrease in the HCHO yield as the pressure was increased (Fig. S3, ESI†).
A previous investigation of CH2I + NO2 at pressures of 2 to 5 Torr gave a value of k8 = (2.2 ± 0.1) × 10−11 cm3 s−1.44 Results of this work show k8 to be (2.56 ± 0.17) × 10−11 cm3 s−1 at 50 Torr, increasing to (5.07 ± 0.28) × 10−11 cm3 s−1 at 300 Torr.
The rate coefficient for reaction of CH2I radicals with O2(R2), has been shown previously to be ∼1.6 × 10−12 cm3 s−1.45,46 Experiments to investigate HCHO production in the reaction of CH2OO (produced by CH2I + O2) with NO2 must therefore be conducted at sufficiently high [O2] to avoid complications owing to HCHO production from CH2I + NO2.
3.4 CH2OO + NO2
Experiments to investigate CH2OO + NO2(R9) kinetics were performed with sufficient NO2 concentrations (1.0 × 1014 to 1.4 × 1015 cm−3) to ensure pseudo-first-order conditions for CH2OO loss whilst also ensuring that k2[O2] > k8[NO2] at all times to avoid potential complications owing to HCHO production through CH2I + NO2.| CH2I2 + hν → CH2I + I | (R1) |
| CH2I + O2 → CH2OO + I | (R2a) |
| CH2I + NO2 → HCHO + products | (R8) |
| CH2OO + NO2 → HCHO + NO3 | (R9) |
Fig. 1 shows the evolution of the HCHO signal following photolysis of CH2I2–O2–N2–NO2 mixtures. Experiments in which NO2 was used as a co-reagent resulted in a decrease in the total HCHO yield when compared to experiments performed in the absence of any co-reagent. We attribute this to the formation of the peroxy nitrate species CH2IO2NO2 which inhibits formation of HCHO through reactions (R4)–(R6).
Experiments performed at 273 K to increase the lifetime of CH2IO2NO2 with respect to dissociation to CH2IO2NO2 did not result in any significant decrease in the HCHO yield compared to equivalent experiments at 295 K, indicating that the CH2IO2NO2 lifetime at 295 K is sufficiently long to minimise production of HCHO from CH2IO2. Thus, while there is a small contribution to the HCHO signal owing to rapid chemistry following multi-photon photolysis of CH2I2, the growth of HCHO observed following photolysis of CH2I2–O2–N2–NO2 mixtures can be attributed to CH2OO + NO2(R9) exclusively.
The pseudo-first-order rate coefficient for the reaction of CH2OO with NO2 was determined by least-squares fitting to eqn (1), with kg′ = k9[NO2]. The bimolecular rate coefficient for CH2OO + NO2 (k9) was subsequently determined from plots of kg′ against [NO2], as shown in Fig. 6. Fits to experimental data using the numerical integration package Kintecus47 to determine k9, detailed in the ESI,† gave results within 10% of those obtained using the analytical expression (eqn (1)).
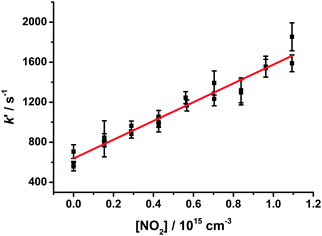 | ||
| Fig. 6 Pseudo-first-order rate coefficients (kg′) for HCHO production at 50 Torr, derived from fits to eqn (1), following photolysis of CH2I2–O2–N2 in the presence of NO2. Error bars are 1σ. The fit to the data (shown in red) gives the bimolecular rate coefficient for CH2OO + NO2 (k9). | ||
Values for k9 as a function of pressure are shown in Fig. 7 and Table 2. No significant dependence of k9 on total pressure was observed over the pressure range investigated (25 to 300 Torr), with an average value of k9 = (1.5 ± 0.5) × 10−12 cm3 s−1. Errors in k9 include the 1σ errors in the fits to the bimolecular plots at each pressure and an error of ±10% to account for any differences between fits using the analytical expression and those obtained by numerical integration (see ESI†).
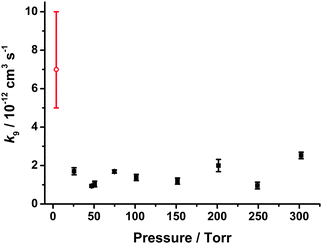 | ||
| Fig. 7 Bimolecular rate coefficients for CH2OO + NO2 (k9) as a function of pressure. Error bars are 1σ. The data point shown by the red open circle is that determined by Welz et al.10 | ||
| Pressure (Torr) | k 9 (10−12 cm3 s−1) | Ref. |
|---|---|---|
| a Measured using N2 as the bath gas. b Measured using O2 as the bath gas. | ||
| 4 | 7+3−2 | Welz et al.10 |
| 25a | 1.70 ± 0.38 | This work |
| 50a | 1.04 ± 0.27 | This work |
| 50b | 0.94 ± 0.16 | This work |
| 75a | 1.69 ± 0.28 | This work |
| 100a | 1.38 ± 0.33 | This work |
| 150a | 1.19 ± 0.30 | This work |
| 200a | 2.00 ± 0.56 | This work |
| 250a | 0.96 ± 0.29 | This work |
| 300a | 2.53 ± 0.47 | This work |
Yields of HCHO in the presence of NO2, determined relative to experiments performed in the absence of NO2 (i.e. production through reactions (R3)–(R6)), were consistent with the yields of CH2OO determined in our previous work26 (Fig. 8). This result demonstrates that ∼100% of CH2OO is titrated to HCHO by CH2OO + NO2, indicating a lack of pressure dependence in k9, and that there is insignificant HCHO production from CH2IO2 in the presence of NO2. Recent measurements by Ouyang et al.21 have demonstrated the production of NO3 at atmospheric pressure from the reaction of CH2OO with NO2, thus also suggesting little stabilisation of reaction products to a secondary ozonide species in this system.
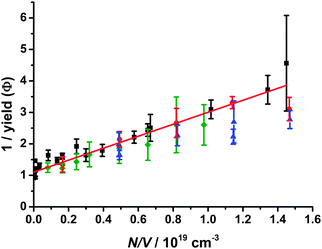 | ||
| Fig. 8 Stern–Volmer plot showing (inverse) yields of CH2OO as a function of pressure from the reaction of CH2I with O2. Results from our previous work are shown for experiments monitoring iodine atom production in the system (black squares), and monitoring of HCHO production in experiments with SO2 (blue triangles) and NO (red circles), with the best fit line (red). Yields of HCHO from the reaction of CH2OO with NO2 (this work, green diamonds), determined relative to the HCHO yields in the absence of NO2 (i.e. through reactions (R3)–(R6)), suggest that there is 100% titration of CH2OO to HCHO in the presence of NO2 at all pressures (i.e. there is no stabilisation of reaction products), and that there is little production of HCHO from CH2IO2 in the system. The fit to our previous work (comprising data from the I atom, NO and SO2 experiments) gives an intercept of 1.10 ± 0.23 and a slope of (1.90 ± 0.22) × 10−19 cm3. The NO2 experiments give an intercept of 1.05 ± 0.12 and a slope of (1.70 ± 0.18) × 10−19 cm3. | ||
No significant difference in k9 or in yields of HCHO were observed between experiments performed in O2 bath gas and N2 bath gas (results shown in Table 2), providing further evidence for similar quenching of the nascent excited CH2IO2# species (produced in (R2)) by O2 and N2, as discussed in our previous work.26
Results for k9 obtained in this work, while lower than those reported by Welz et al.,10 are on the same order of magnitude, and demonstrate a significantly faster reaction between CH2OO and NO2 than suggested by previous indirect measurements.1
3.5 CH2OO + NO
Production of HCHO following photolysis of CH2I2–O2–N2 mixtures in the presence of excess NO (3.6 × 1014 to 1.7 × 1015 cm−3) exhibits biexponential growth, as shown in Fig. 9, similar to experiments with SO2. Again, no decrease in the total HCHO yield compared to experiments performed in the absence of any co-reagent, indicating complete titration of both CH2OO and CH2IO2 to HCHO. Kinetic parameters for the processes contributing to HCHO production were obtained by fitting to eqn (3) (above).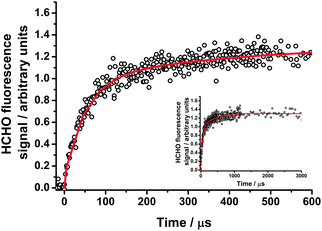 | ||
Fig. 9 HCHO fluorescence signals at 250 Torr following photolysis of CH2I2–O2–N2 in the presence of NO, with the fit to eqn (3) (solid red lines). The inset panel shows the evolution of the signal to longer times. For these data, kg1′ = (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 ± 1400) s−1; kg2′ = (2660 ± 320) s−1; kloss = (10 ± 2) s−1; f = (0.70 ± 0.02); S1 = (1.33 ± 0.01). 800 ± 1400) s−1; kg2′ = (2660 ± 320) s−1; kloss = (10 ± 2) s−1; f = (0.70 ± 0.02); S1 = (1.33 ± 0.01). | ||
The rate coefficient describing the fast HCHO growth process, kg1′, was observed to increase linearly with increasing [NO], with the slope of a plot of kg1′ against [NO] giving a bimolecular rate coefficient of (1.07 ± 0.06) × 10−11 cm3 s−1 at 250 Torr (Fig. S4, ESI†). The rate coefficient describing the slower HCHO growth, kg2′, was found to be independent of [NO], and similar to the rate coefficient for HCHO production obtained in the absence of NO. Reactions of peroxy radicals (RO2) with NO are well established, and are typically on the order of 10−12 to 10−11 cm3 s−1,48,49 with a rate coefficient for CH3O2 + NO of 7.2 × 10−12 cm3 s−1,49 while Welz et al.10 reported an upper limit of 6 × 10−14 cm3 s−1 for the rate coefficient for CH2OO with NO. Thus, in contrast to the experiments with SO2, we attribute the fast HCHO growth to the rapid decomposition of CH2IO (R6), produced in the reaction of CH2IO2 with NO (R10) and assign k10 = (1.07 ± 0.06) × 10−11 cm3 s−1 at 250 Torr.
| CH2IO2 + NO → CH2IO + NO2 | (R10) |
| CH2IO → HCHO + I | (R6) |
The slower HCHO growth thus contains contributions from CH2OO + I (R3) and potentially CH2OO + NO (R11). In the absence of NO, production of HCHO was observed with a pseudo-first-order rate coefficient of 1860 ± 100 s−1 (eqn (1)). On addition of up to 1.7 × 1015 cm−3 NO, the average value for the rate coefficient describing the slow HCHO growth (kg2′ in eqn (3)) was 1800 ± 340 s−1. Any potential influence of NO on the observed rates of HCHO production is assumed to be within the error of the experiment, and we thus place an upper limit of 2 × 10−13 cm3 s−1 on the rate coefficient for reaction of CH2OO + NO (k11).
| CH2OO + NO → HCHO + NO2 | (R11) |
The upper limit for k11 determined here is higher than that reported by Welz et al. (k11 < 6 × 10−14 cm3 s−1), owing to increased uncertainties associated with the biexponential fit, relatively low concentrations of NO, and higher concentrations of CH2I2 used in these experiments compared to those performed by Welz et al., which lead to increased iodine atom concentrations in this work and thus increased rates of HCHO production through CH2OO + I (R3). In subsequent experiments (notably those used to investigate the kinetics of CH2OO + H2O) lower CH2I2 concentrations were used by changing the delivery method for CH2I2. There are also additional uncertainties in the rate coefficients for reactions with NO owing to the potential for production of NO2 in the gas lines leading to the reaction cell through oxidation of NO by O2 (the gas mixture has a residence time of ∼1 s in the gas lines leading from the mixing line to the reaction cell), leading to the potential for contributions to the observed HCHO growth from reactions involving NO2.
3.6 CH2OO + H2O
Welz et al. did not observe any change in the rate of CH2OO decay on addition of water vapour to the system, and reported an upper limit of 4 × 10−15 cm3 s−1 for the rate coefficient for reaction of CH2OO with H2O (R12):| CH2OO + H2O → HCHO + H2O2 | (R12) |
Similarly to the results of Welz et al., the addition of water vapour to the LIF experiments in this work did not result in any significant change to the rate of HCHO production. The total HCHO yield was also unaffected by the presence of water vapour, indicating complete titration of CH2OO and CH2IO2 to HCHO through reactions (R3)–(R6). Fig. 10 shows the HCHO fluorescence signals following photolysis of CH2I2–O2–N2 in the absence and presence of water vapour. While the HCHO signal is reduced in the presence of water vapour, there is no change in the kinetics and the reduction in signal is attributed to increased fluorescence quenching by water vapour.
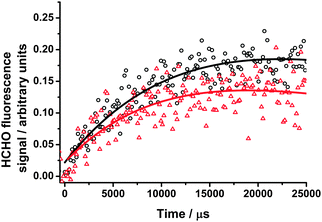 | ||
| Fig. 10 HCHO fluorescence signals at 200 Torr following photolysis of CH2I2–O2–N2 in the absence (black open circles) and presence of water vapour (red open triangles), with the fits to eqn (1) (solid lines). The differences in the amplitude of the signal result from the quenching of the fluorescence signal by H2O. For these data, kg′ = (41 ± 15) s−1 in the absence of water vapour and kg′ = (52 ± 13) s−1 in the presence of water vapour. | ||
At 200 Torr the pseudo-first-order rate coefficient for HCHO production was determined to be 41 ± 15 s−1 by fitting to eqn (1), and was lower than the typical values reported in Section 3.1 as a result of lower concentrations of CH2I2 to reduce the rate of HCHO production through radical–radical reactions in the absence of water vapour. On addition of up to 1.7 × 1017 cm−3 water vapour to the system, a value of 52 ± 13 s−1 was obtained, with no obvious dependence on the concentration of water vapour added. Owing to the higher total pressures used in this work, enabling the addition of a higher number density of water vapour to the system compared to the low pressure experiments by Welz et al., we are able to place an upper limit of 9 × 10−17 cm3 s−1 on k12 at 295 K by assuming any influence of water vapour is within the error of the experiment. Ouyang et al.21 have reported a value for k12 of (2.5 ± 1) × 10−17 cm3 s−1 at 760 Torr, determined in a relative rate experiment monitoring NO3 production and using the absolute value for k9 (CH2OO + NO2) reported by Welz et al.10 (7 × 10−12 cm3 s−1). Using the relative rate coefficient ratio reported by Ouyang et al., with the value for k9 determined in this work (1.5 × 10−12 cm3 s−1), a value of k12 = 5.4 × 10−18 cm3 s−1 can be obtained.
Modelling studies investigating the impacts of CH2OO chemistry on the atmospheric oxidation of SO2 may therefore be underestimating the effects of increasing the rate coefficient for CH2OO + SO2 owing to overestimation of the competition with CH2OO + H2O, resulting in more significant impacts on atmospheric production of H2SO4 and sulfate aerosol than indicated thus far. However, Taatjes et al.12 have shown that the anti-CH3CHOO Criegee intermediate does react with water vapour (k = (1.0 ± 0.4) × 10−14 cm3 s−1), and the lack of reaction between CH2OO and water vapour may not be representative of all Criegee intermediates. Modelling of Criegee chemistry in forested regions in Finland and Germany has indicated that concentration of the CH2OO Criegee intermediate is only ∼20–33% of the concentrations of larger Criegee intermediates derived from monoterpenes,14 with global modelling indicating that the production rate of CH2OO comprises ∼40% of the total global production rate of all Criegee intermediates.15 The chemistry of larger Criegee intermediates warrants further attention.
3.7 CH2OO + CH3CHO
The reactions of Criegee intermediates with carbonyl compounds are of interest not only for their potential atmospheric relevance, but also to facilitate the use of carbonyl compounds as scavengers of Criegee intermediates in alkene ozonolysis experiments, enabling the determination of product yields of ozonolysis reactions.Horie et al.50 studied the relative rates of CH2OO reactions with CH3CHO (R13) and CF3COCF3(R14) at 730 Torr in synthetic air using FT-IR spectroscopy to monitor the decay of CF3COCF3 and the production of the secondary ozonide propene ozonide (methyl-1,2,4-trioxolane) from the reaction with CH3CHO, and found the reaction with CF3COCF3 to be 13 times faster than that with CH3CHO.
| CH2OO + CH3CHO → products | (R13) |
| CH2OO + CF3COCF3 → products | (R14) |
Secondary ozonide products were observed by Horie et al. for both (R13) and (R14) at 730 Torr, while photoionisation mass spectrometry experiments by Taatjes et al.11 at 4 Torr observed a secondary ozonide product for (R14) but not for (R13). Absolute rate coefficients for CH2OO + CH3CHO and CH2OO + CF3COCF3 were measured by Taatjes et al.11 at 4 Torr in He by direct monitoring of CH2OO, with results indicating the reaction with CF3COCF3 to be ∼32 times faster than that with CH3CHO and k13 = (9.4 ± 0.7) × 10−13 cm3 s−1 at 4 Torr. As discussed by Taatjes et al.,11 the differences between the results of Horie et al. and Taatjes et al. may arise from differences in the fall-off behaviour of the two reactions, indicating pressure dependence of one or both of the reactions over the range of pressures investigated. Differences in product observations between the two studies also suggest pressure dependence in k13. In the low pressure experiments, Taatjes et al. do not observe formation of secondary ozonide products. At 730 Torr, propene ozonide was observed as the major product of (R13), indicating collisional stabilisation of the nascent secondary ozonide at high pressures. Recent theoretical work22 has investigated the potential energy surface for the reaction of CH2OO with CH3CHO, and supports the observed pressure dependence of the reaction. Reaction products are predicted to be collisionally stabilised to a secondary ozonide (SOZ) species, with significant production of the SOZ at atmospheric pressure (760 Torr) and the SOZ dominating the reaction products at pressures above 1000 Torr.
Pressure dependent kinetics are expected to be typical for reactions of larger Criegee intermediates with atmospherically relevant species, including SO2, and investigation of the CH2OO + CH3CHO system may therefore provide insight to the behaviour of other Criegee intermediates.
In this work, we investigate HCHO production from CH2OO + CH3CHO (R13) at total pressures between 25 and 300 Torr and concentrations of CH3CHO in the range 2 × 1014 to 1 × 1015 cm−3. Production of HCHO displayed single exponential growth, and the HCHO fluorescence signal was fitted to eqn (1) (Fig. 11). Fig. 12 shows the bimolecular plot used to determine k13 at 25 Torr, giving k13 = (1.48 ± 0.04) × 10−12 cm3 s−1 at 25 Torr. The HCHO yield from (R13) (corrected for any HCHO production from CH2IO2 in reactions (R4)–(R6) using the results of our previous work) was observed to decrease with increasing pressure, indicating stabilisation of the CH2OO + CH3CHO reaction product at higher pressures (R13b) and pressure dependence in k13.
| CH2OO + CH3CHO → CH2OO–CH3CHO# |
| CH2OO–CH3CHO# → HCHO + CH3C(O)OH | (R13a) |
| CH2OO–CH3CHO# + M → propene ozonide + M | (R13b) |
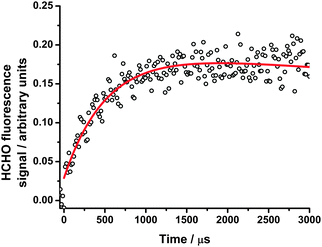 | ||
| Fig. 11 HCHO fluorescence signals at 25 Torr following photolysis of CH2I2–O2–N2 in the presence of CH3CHO, with the fit to eqn (1) (solid red line). For these data, kg′ = (2040 ± 120) s−1. | ||
Fig. 13 shows the Stern–Volmer plot for HCHO yields from (R13), giving an intercept of 1.19 ± 0.39 and slope (k13b/k13a) of (1.09 ± 0.08) × 10−18 cm3. Using an intercept of 1, at 4 Torr we estimate a yield of HCHO of 88%, with a yield of 4% at 730 Torr, reconciling the results of Taatjes et al.11 and Horie et al.50 and in agreement with theoretical work of Jalan et al.22
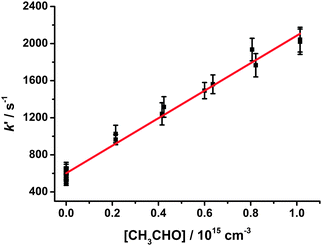 | ||
| Fig. 12 Pseudo-first-order rate coefficients (kg′) for HCHO production at 25 Torr, derived from fits to eqn (1), following photolysis of CH2I2–O2–N2 in the presence of CH3CHO. Error bars are 1σ. The fit to the data (shown in red) gives the bimolecular rate coefficient for CH2OO + CH3CHO (k13). | ||
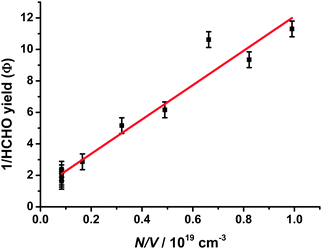 | ||
| Fig. 13 Stern–Volmer analysis for HCHO yields from CH2OO + CH3CHO (R13) (corrected for HCHO production from CH2IO2 chemistry) as a function of total pressure, with the fit to the data (red). Error bars are 1σ. | ||
Owing to the decrease in HCHO yield with increasing pressure, assignment of the kinetics of (R13) at pressures above 25 Torr is challenging. Using the results of Taatjes et al.11 at 4 Torr (k13 = (9.5 ± 0.7) × 10−13 cm3 s−1), together with those determined here at 25 Torr (k13 = (1.48 ± 0.04) × 10−12 cm3 s−1), 50 Torr (∼2.2 × 10−12 cm3 s−1) and the determination of k13b/k13a from the Stern–Volmer plot ((1.09 ± 0.08) × 10−18 cm3), we estimate a low pressure limit (k13,0) of ∼1.6 × 10−29 cm6 s−1 and a high pressure limit (k13,∞) of ∼1.7 × 10−12 cm3 s−1 (see ESI†).
4. Conclusions
Reactions of the CH2OO Criegee intermediate with NO2, NO, SO2, H2O and CH3CHO have been investigated over a range of pressures. The reactions of CH2OO with NO2, SO2 and CH3CHO are rapid, in agreement with recent measurements by Welz et al.10 and Taatjes et al.11 but in contrast to recommendations for atmospheric modelling based on indirect measurements. Rate coefficients for reactions of CH2OO with NO2 and SO2 are essentially independent of pressure over the pressure ranges studied in this work. The rate coefficient for CH2OO + CH3CHO is pressure dependent, with stabilisation to form the secondary ozonide reaction products at high pressures.We observe no evidence for reactions of CH2OO with NO or H2O under the conditions employed in this work, and place upper limits on rate coefficients for these reactions of 2 × 10−13 cm3 s−1 and 9 × 10−17 cm3 s−1, respectively. The upper limit for the rate coefficient for CH2OO + H2O is significantly lower than has been reported previously. Earlier assessments2,14,15,17 of the impacts of increased reaction rates for CH2OO + SO2 and CH2OO + NO2 will therefore be lower limits owing to overestimation of the impacts of CH2OO + H2O.
Acknowledgements
The authors are grateful to the National Centre for Atmospheric Science (NCAS) and the Engineering and Physical Sciences Research Council (EPSRC, grant reference EP/J010871/1) for funding.References
- D. Johnson and G. Marston, Chem. Soc. Rev., 2008, 37, 699–716 RSC.
- C. A. Taatjes, D. E. Shallcross and C. Percival, Phys. Chem. Chem. Phys., 2013 10.1039/C3CP52842A.
- N. M. Donahue, G. T. Drozd, S. A. Epstein, A. A. Presto and J. H. Kroll, Phys. Chem. Chem. Phys., 2011, 13, 10848–10857 RSC.
- M. S. Alam, M. Camredon, A. R. Rickard, T. Carr, K. P. Wyche, K. E. Hornsby, P. S. Monks and W. J. Bloss, Phys. Chem. Chem. Phys., 2011, 12, 11002–11015 RSC.
- L. Vereecken, Science, 2013, 340, 154–155 CrossRef CAS PubMed.
- T. L. Malkin, A. Goddard, D. E. Heard and P. W. Seakins, Atmos. Chem. Phys., 2010, 10, 1441–1459 CrossRef CAS.
- D. E. Heard, L. J. Carpenter, D. J. Creasey, J. R. Hopkins, J. D. Lee, A. C. Lewis, M. J. Pilling, P. W. Seakins, N. Carslaw and K. M. Emmerson, Geophys. Res. Lett., 2004, 31, L18112 CrossRef.
- R. M. Harrison, J. Yin, R. M. Tilling, X. Cai, P. W. Seakins, J. R. Hopkins, D. L. Lansley, A. C. Lewis, M. C. Hunter, D. E. Heard, L. J. Carpenter, D. J. Creasey, J. D. Lee, M. J. Pilling, N. Carslaw, K. M. Emmerson, A. Redington, R. G. Derwent, D. Ryall, G. Mills and S. A. Penkett, Sci. Total Environ., 2006, 360, 5–25 CrossRef CAS PubMed.
- D. Stone, L. K. Whalley and D. E. Heard, Chem. Soc. Rev., 2012, 41, 6348–6404 RSC.
- O. Welz, J. D. Savee, D. L. Osborn, S. S. Vasu, C. J. Percival, D. E. Shallcross and C. A. Taatjes, Science, 2012, 335, 204–207 CrossRef CAS PubMed.
- C. A. Taatjes, O. Welz, A. J. Eskola, J. D. Savee, D. L. Osborn, E. P. F. Lee, J. M. Dyke, D. W. K. Mok, D. E. Shallcross and C. J. Percival, Phys. Chem. Chem. Phys., 2012, 14, 10391–10400 RSC.
- C. A. Taatjes, O. Welz, A. J. Eskola, J. D. Savee, A. M. Scheer, D. E. Shallcross, B. Rotavera, E. P. F. Lee, J. M. Dyke, D. K. W. Mok, D. L. Osborn and C. J. Percival, Science, 2013, 340, 177–180 CrossRef CAS PubMed.
- L. Vereecken, H. Harder and A. Novelli, Phys. Chem. Chem. Phys., 2012, 14, 14682–14695 RSC.
- M. Boy, D. Mogensen, S. Smolander, L. Zhou, T. Nieminen, P. Paasonen, C. Plass-Duelmer, M. Sipila, T. Petaja, L. Mauldin, H. Berresheim and M. Kulmala, Atmos. Chem. Phys., 2013, 13, 3865–3879 CrossRef CAS.
- J. R. Pierce, M. J. Evans, C. E. Scott, S. D. D'Andrea, D. K. Farmer, E. Swietlicki and D. V. Spracklen, Atmos. Chem. Phys., 2013, 13, 3163–3176 CrossRef CAS.
- G. Sarwar, K. Fahey, R. Kwok, R. C. Gilliam, S. J. Roselle, R. Mathur, J. Xue, J. Yu and W. P. L. Carter, Atmos. Environ., 2013, 68, 186–197 CrossRef CAS PubMed.
- C. J. Percival, O. Welz, A. J. Eskola, J. D. Savee, D. L. Osborn, D. O. Topping, D. Lowe, S. R. Utembe, A. Bacak, G. McFiggans, M. C. Cooke, P. Xiao, A. T. Archibald, M. E. Jenkin, R. G. Derwent, I. Riipinen, D. W. K. Mok, E. P. F. Lee, J. M. Dyke, C. A. Taatjes and D. E. Shallcross, Faraday Discuss., 2013 10.1039/C3FD00048F.
- C. A. Taatjes, G. Meloni, T. M. Selby, A. J. Trevitt, D. L. Osborn, C. J. Percival and D. E. Shallcross, J. Am. Chem. Soc., 2008, 130, 11883–11885 CrossRef CAS PubMed.
- Y.-T. Su, Y.-H. Huang, H. A. Witek and Y.-P. Lee, Science, 2013, 340, 174–176 CrossRef CAS PubMed.
- J. M. Beames, F. Liu, L. Lu and M. I. Lester, J. Am. Chem. Soc., 2012, 134, 20045–20048 CrossRef CAS PubMed.
- B. Ouyang, M. W. McLeod, R. L. Jones and W. J. Bloss, Phys. Chem. Chem. Phys., 2013, 15, 17070–17075 RSC.
- A. Jalan, J. W. Allen and W. H. Green, Phys. Chem. Chem. Phys., 2013, 15, 16841–16852 RSC.
- R. L. Mauldin, III, T. Berndt, M. Sipilae, P. Paasonen, T. Petaja, S. Kim, T. Kurten, F. Stratmann, V. M. Kerminen and M. Kulmala, Nature, 2012, 488, 193–197 CrossRef PubMed.
- T. Kurten, J. R. Lane, S. Jorgensen and H. G. Kjaergaard, J. Phys. Chem. A, 2011, 115, 8669–8681 CrossRef CAS PubMed.
- H. Huang, A. J. Eskola and C. A. Taatjes, J. Phys. Chem. Lett., 2012, 3, 3399–3403 CrossRef CAS.
- D. Stone, M. Blitz, L. Daubney, T. Ingham and P. Seakins, Phys. Chem. Chem. Phys., 2013, 15, 19119–19124 RSC.
- H. Huang, B. Rotavera, A. J. Eskola and C. A. Taatjes, J. Phys. Chem. Lett., 2013, 4, 3824 CrossRef CAS.
- C. M. Roehl, J. B. Burkholder, G. K. Moortgat, A. R. Ravishankara and P. Crutzen, J. Geophys. Res.: Atmos., 1997, 102, 12819–12829 CrossRef CAS.
- L. J. Carpenter, W. T. Sturges, S. A. Penkett, P. S. Liss, B. Alicke, K. Hebestreit and U. Platt, J. Geophys. Res., [Atmos.], 1999, 104, 1679–1689 CrossRef CAS.
- L. J. Carpenter, Chem. Rev., 2003, 103, 4953–4962 CrossRef CAS PubMed.
- L. J. Carpenter, S. D. Archer and R. Beale, Chem. Soc. Rev., 2012, 41, 6473–6506 RSC.
- T. J. Gravestock, M. A. Blitz, W. J. Bloss and D. E. Heard, ChemPhysChem, 2010, 11, 3928–3941 CrossRef CAS PubMed.
- R. A. Cox, J. Phys. Chem., 1972, 76, 814–820 CrossRef CAS.
- J. L. Jourdain, G. Lebras and J. Combourieu, Int. J. Chem. Kinet., 1979, 11, 569–577 CrossRef CAS.
- K. J. Hughes, M. A. Blitz, M. J. Pilling and S. H. Robertson, Proc. Combust. Inst., 2002, 29, 2431–2437 CrossRef CAS.
- D. T. Co, T. F. Hanisco, J. G. Anderson and F. N. Keutsch, J. Phys. Chem. A, 2005, 109, 10675–10682 CrossRef CAS PubMed.
- M. A. Blitz, A. Goddard, T. Ingham and M. J. Pilling, Rev. Sci. Instrum., 2007, 78, 034103 CrossRef PubMed.
- M. Teresa Baeza-Romero, M. A. Blitz, A. Goddard and P. W. Seakins, Int. J. Chem. Kinet., 2012, 44, 532–545 CrossRef.
- G. Hancock and V. Haverd, Chem. Phys. Lett., 2003, 372, 288–294 CrossRef CAS.
- H. M. Su, W. T. Mao and F. N. Kong, Chem. Phys. Lett., 2000, 322, 21–26 CrossRef CAS.
- U. Bley, F. Temps, H. G. Wagner and M. Wolf, Ber. Bunsen-Ges. Phys. Chem., 1992, 96, 1043–1048 CAS.
- R. A. Alvarez and C. B. Moore, J. Phys. Chem., 1994, 98, 174–183 CrossRef CAS.
- M. A. Blitz, C. Kappler, M. J. Pilling and P. W. Seakins, Z. Phys. Chem., 2011, 225, 957–967 CrossRef CAS.
- A. J. Eskola, D. Wojcik-Pastuszka, E. Ratajczak and R. S. Timonen, J. Phys. Chem. A, 2006, 110, 12177–12183 CrossRef CAS PubMed.
- A. J. Eskola, D. Wojcik-Pastuszka, E. Ratajczak and R. S. Timonen, Phys. Chem. Chem. Phys., 2006, 8, 1416–1424 RSC.
- A. Masaki, S. Tsunashima and N. Washida, J. Phys. Chem., 1995, 99, 13126–13131 CrossRef CAS.
- J. C. Ianni, Kintecus, Windows Version 2.80, www.kintecus.com, 2002 Search PubMed.
- P. D. Lightfoot, R. A. Cox, J. N. Crowley, M. Destriau, G. D. Hayman, M. E. Jenkin, G. K. Moortgat and F. Zabel, Atmos. Environ., Part A, 1992, 26, 1805–1961 CrossRef.
- R. Atkinson, D. L. Baulch, R. A. Cox, J. N. Crowley, R. F. Hampson, R. G. Hynes, M. E. Jenkin, M. J. Rossi and J. Troe, Atmos. Chem. Phys., 2006, 6, 3625–4055 CrossRef CAS.
- O. Horie, C. Schafer and G. K. Moortgat, Int. J. Chem. Kinet., 1999, 31, 261–269 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cp54391a |
| This journal is © the Owner Societies 2014 |
