Absolute thermodynamic properties of molten salts using the two-phase thermodynamic (2PT) superpositioning method
Abstract
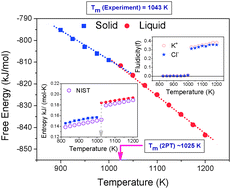
We show that the absolute thermodynamic properties of molten salts (mixtures of KCl and LiCl) can be accurately determined from the two-phase thermodynamic (2PT) method that is based on superpositioning of solid-like and gas-like (hard-sphere) vibrational density of states (DoS). The 2PT predictions are in excellent accordance with those from the thermodynamic integration method; the melting point of KCl evaluated from the free energy and the absolute entropy shows close conformity with the experimental/NIST data. The DoS partitioning shows that the Li+ ions in the eutectic LiCl–KCl molten mixture are largely solid-like, unlike the K+ and Cl− ions, which have a significant gas-like contribution, for temperatures ranging from 773 K to 1300 K. The solid-like states of the Li+ ions may have practical implications when employed for chemical and nuclear reprocessing applications.

 Please wait while we load your content...
Please wait while we load your content...