Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A 3-dimensional {42·84} lvt net built from a ditopic bis(3,2′:6′,3″-terpyridine) tecton bearing long alkyl tails†
Y.
Maximilian Klein
,
Edwin C.
Constable
,
Catherine E.
Housecroft
* and
Alessandro
Prescimone
Department of Chemistry, University of Basel, Spitalstrasse 51, CH4056 Basel, Switzerland. E-mail: catherine.housecroft@unibas.ch; Fax: +41 61 267 1018; Tel: +41 61 267 1008
First published on 16th December 2014
Abstract
Divergent bis(terpyridine) tectons are versatile ligands for the assembly of coordination networks; we demonstrate the assembly of a 3-dimensional {42·84} lvt net (still relatively sparse among 4-connected nets in metal–organic frameworks) from the reaction of 1,4-bis(n-octoxy)-2,5-bis(3,2′:6′,3″-terpyridin-4′-yl)benzene and Co(NCS)2.
Oligopyridines1 remain as one of the most widespread building blocks in the toolbox of a coordination chemist. The bis(chelate) 2,2′:6′,2″-terpyridine (2,2′:6′,2″-tpy) is especially popular and multitopic ligands containing peripheral 2,2′:6′,2″-tpy domains2 have been used in a wide variety of architectures. 4,2′:6′,4″-Terpyridine (4,2′:6′,4″-tpy, Scheme 1) and 3,2′:6′,3″-terpyridine (3,2′:6′,3″-tpy, Scheme 1) are less familiar isomers of terpyridine, but in the last decade, the use of 4,2′:6′,4″-tpy as a building block of coordination polymers has grown significantly.3 In metal complexes of 4,2′:6′,4″-tpy, the central pyridine ring is non-coordinated and the remaining N,N′-donor set presents a divergent domain, with vectorial properties that are unaffected by inter-ring bond rotation (red bonds in Scheme 1). In contrast, rotation about the interannular bonds in 3,2′:6',3″-tpy alters its divergent coordination mode.4
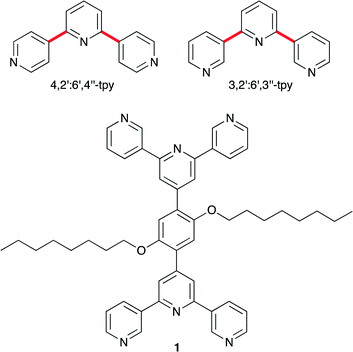 | ||
| Scheme 1 Structures of divergent isomers of terpyridine and the ditopic ligand 1. See text for significance of the bonds marked in red. | ||
The beauty of terpyridine metal-binding domains is the ease with which substituents can be introduced into the 4′-position, for example by using Kröhnke's5 or Wang and Hanan's6 strategies. With coordinatively innocent 4′-substituents, reactions between 4,2′:6′,4″-terpyridines and metal ions yield metallomacrocycles, 1-dimensional chains or 2-dimensional nets.3 Extension to 3-dimensions is achieved by introducing non-innocent domains such as diphenylphosphino,7 carboxylato,8 or pyridyl9 functionalities, or by using co-ligands.10 An alternative approach to increase dimensionality is through the coordination capacity of multitopic 4,2′:6′,4″-tpy ligands, although such compounds have received scant attention.11–14 We have demonstrated that 1,4-bis(n-octoxy)-2,5-bis(4,2′:6′,4″-terpyridin-4′-yl)benzene reacts with ZnCl2 to give a network consisting of (4,4)-sheets engaging in 2D → 2D parallel interpenetration,14 and a report on a triply interpenetrating network formed between cobalt(II) and 1,3-di((4,2′:6′,4″-terpyridin)-4′-yl)benzene has appeared.12 We now describe the synthesis of the bis(3,2′:6′,3″-tpy) ligand 1 (Scheme 1) and its reaction with Co(NCS)2 to give a 3-dimensional {42·84} lvt net.15 The inclusion of the long alkoxy chains in 1 enhances the solubility of the ligand with respect to analogues with simple phenylene spacers,14 and also has a stabilizing influence on an infinite architecture.
Ligand 1 was synthesized† using the one-pot method of Wang and Hanan6 starting from 2,5-bis(octoxy)benzene-1,4-dicarbaldehyde and 4.7 equivalents of 3-acetylpyridine in EtOH in the presence of NH3. Compound 1 was isolated in 41% yield. The electrospray mass spectrum exhibited a base peak at m/z 797.8 arising from [M+H]+, and 1H (Fig. S1†) and 13C NMR spectra (assigned using COSY, DEPT, HMQC and HMBC techniques) were consistent with the symmetrical structure shown in Scheme 1. The absorption spectrum of 1 has broad and intense, high energy bands arising from π* ← n and π* ← π transitions which extend into the visible region (Fig. S2†).
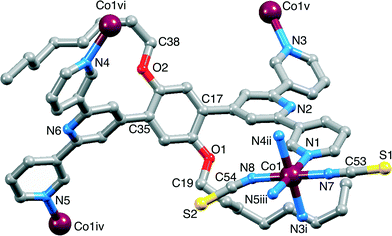
Layering of MeOH and CHCl3 solutions of Co(NCS)2 and 1, respectively, resulted in the formation of pink crystals of [Co(NCS)2(1)·4CHCl3]n within 2–4 weeks in 14% yield. The crystal selected for single crystal X-ray diffraction was solved and refined in the non-centrosymmetric space group Pna21. The Flack parameter of 0.480(10) suggested that it was a twin by inversion16 as every attempt to either solve or refine the structure in Pnma failed and ADDSYMM17 could not identify an alternative space group. The asymmetric unit contains one molecule of 1 and a Co(NCS)2 unit; the octoxy chain containing atom C19 was refined isotropically and three of the C–C distances were restrained to chemically reasonable values. The cobalt ion is octahedrally coordinated, and 1 binds through the outer N-donors to four cobalt centres (Fig. 1), leaving the central nitrogen atoms N2 and N6 uncoordinated. Atom Co1 coordinates to two [NCS]− ligands in a trans-arrangement and to four different 1 ligands in the equatorial plane. The two independent octoxy chains are in non-extended conformation, and each is folded over a 3,2′:6′,3″-tpy unit with close C–H⋯π contacts (H⋯centroid = 3.19 and 3.23 Å for the pyridine rings containing N1 and N4).
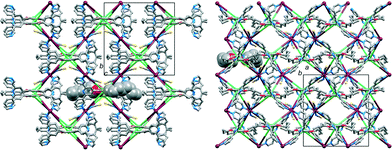
Pairs of cobalt atoms are either bridged by a single 4,2′:6′,4″-tpy (e.g. Co1 and Co1v in Fig. 1) or by two N-donors from each of the two 4,2′:6′,4″-tpy domains of 1 (e.g. Co1 and Co1iv in Fig. 1). The latter coordination mode extends to the formation of [2 + 2] metallomacrocycles (Fig. 2a) which are interlinked through the cobalt centres, as shown in Fig. 2b. The structure propagates into a 2-nodal {42·84} lvt net.14 The two 4-connected nodes are Co1 and the centroid of the arene spacer in 1, which are planar and approximately tetrahedral, respectively. Although the local {Co(Ntpy)4} domain in [Co(NCS)2(1)·4CHCl3]n is square planar (Ntpy–Co–Ntpy angles = 90.9(3), 89.1(3), 92.9(3) and 87.1(3)°), the cobalt node is distorted in the topological description of the net (centroid–Co–centroid angles = 96.6, 64.6, 132.2 and 67.4°) while remaining planar. Topological representations of the framework in [Co(NCS)2(1)·4CHCl3]n are shown in Fig. 3. The view down the a-axis in Fig. 3a is directly comparable with the structure in Fig. 2b.
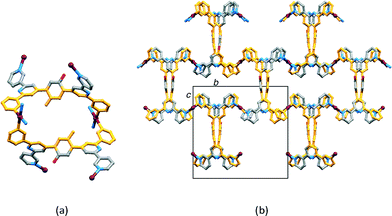 | ||
| Fig. 2 (a) A [2 + 2] metallomacrocycle formed from two Co1 atoms and two half-ligands (in orange). (b) Interconnection of metallomacrocycles with the unit cell viewed down the a-axis. | ||
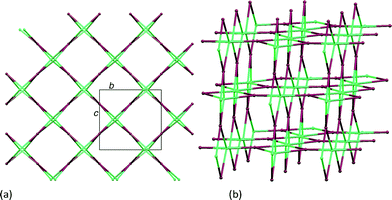 | ||
| Fig. 3 TOPOS18 representations of the {42·84} lvt net in [Co(NCS)2(1)·4CHCl3]n: (a) view down the a-axis for comparison with Fig. 2b, and (b) showing the 4- and 8-membered metallomacrocycles. Metal nodes, purple; ligand–centroid nodes, green. | ||
The voids in the net are occupied by the octoxy chains and CHCl3 molecules. Fig. 4 illustrates that the chains lie in the ac-plane, a consequence of the close CH⋯π contacts between the terminal CH2CH3 units of each chain and pyridine rings (see above).
In conclusion, we have shown that, by adopting a conformation in which the two 3,2′:6′,3″-tpy metal-binding domains are orthogonal, ligand 1 can combine with a planar 4-connecting metal node to produce a {42·84} lvt net. Among metal–organic frameworks, the lvt topology is scarce in comparison with other 4-connected nets.19 The conformational flexibility of ditopic bis(4,2′:6′,4″-tpy) and bis(3,2′:6′,3″-tpy) ligands allows the two tpy units to lie on a path between coplanar13 and orthogonal (as in the current work), making these isomeric ligands attractive tectons. We are currently developing the coordination chemistry of multitopic 4,2′:6′,4″-tpy and 3,2′:6′,3″-tpy ligands to investigate which building blocks favour the assembly of 2- versus 3-dimensional networks.
Acknowledgements
We thank the Swiss National Science Foundation (grant 200020_149067), the European Research Council (Advanced grant 267816 LiLo) and the University of Basel for financial support.Notes and references
- R. P. Thummel, Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Oxford, 2003, vol. 1, p. 41 Search PubMed.
- See for example: E. C. Constable, Chem. Soc. Rev., 2007, 36, 246 RSC; E. C. Constable, Coord. Chem. Rev., 2008, 252, 842 CrossRef CAS PubMed; E. C. Constable, A. M. W. Cargill Thompson, P. Harveson, L. Macko and M. Zehnder, Chem. – Eur. J., 1995, 1, 360 CrossRef; A. Wild, A. Winter, F. Schluetter and U. S. Schubert, Chem. Soc. Rev., 2011, 40, 1459 RSC; Y. Yan and J. Huang, Coord. Chem. Rev., 2010, 254, 1072 CrossRef PubMed; E. C. Constable, Chimia, 2013, 67, 388 CrossRef PubMed , and references therein and references therein.
- C. E. Housecroft, Dalton Trans., 2014, 43, 6594 RSC.
- E. C. Constable, C. E. Housecroft, M. Neuburger, S. Vujovic, J. A. Zampese and G. Zhang, CrystEngComm, 2012, 14, 3554 RSC.
- F. Kröhnke, Synthesis, 1976, 1 CrossRef.
- J. Wang and G. S. Hanan, Synlett, 2005, 1251 CAS.
- X. Tan, X. Chen, J. Zhang and C.-Y. Song, Dalton Trans., 2012, 41, 3616 RSC.
- See for example: L. Wen, X. Ke, L. Qiu, Y. Zou, L. Zhou, J. Zhao and D. Li, Cryst. Growth Des., 2012, 12, 4083 Search PubMed; C. Niu, A. Ning, C. Feng, X. Wan and C. Kou, J. Inorg. Organomet. Polym., 2012, 22, 519 CrossRef CAS; P. Yang, M.-S. Wang, J.-J. Shen, M.-X. Li, Z.-X. Wang, M. Shao and X. He, Dalton Trans., 2014, 43, 1460 RSC; Y.-L. Gai, F.-L. Jiang, L. Chen, Y. Bu, M.-Y. Wu, K. Zhou, J. Pan and M.-C. Hong, Dalton Trans., 2013, 42, 9954 RSC; Y. Li, Z. Ju, B. Wu and D. Yuan, Cryst. Growth Des., 2013, 13, 4125 Search PubMed; F. Yuan, J. Xie, H.-M. Hu, C.-M. Yuan, B. Xu, M.-L. Yang, F.-X. Dong and G.-L. Xue, CrystEngComm, 2013, 15, 1460 RSC; F. Yuan, Q. Zhu, H.-M. Hu, J. Xie, B. Xu, C.-M. Yuan, M.-L. Yang, F.-X. Dong and G.-L. Xue, Inorg. Chim. Acta, 2013, 397, 117 CrossRef PubMed; H.-N. Zhang, F. Yuan, H.-M. Hu, S.-S. Shen and G.-L. Xue, Inorg. Chem. Commun., 2013, 34, 51 CrossRef PubMed; W. Yang, A. J. Davies, X. Lin, M. Suyetin, R. Matsuda, A. J. Blake, C. Wilson, W. Lewis, J. E. Parker, C. C. Tang, M. W. George, P. Hubberstey, S. Kitagawa, H. Sakamoto, E. Bichoutskaia, N. R. Champness, S. Yang and M. Schröder, Chem. Sci., 2012, 3, 2992 Search PubMed; Y.-Q. Chen, S.-J. Liu, Y.-W. Li, G.-R. Li, K.-H. He, Y.-K. Qu, T.-L. Hu and X.-H. Bu, Cryst. Growth Des., 2012, 12, 5426 Search PubMed; F. Yuan, J. Xie, H.-M. Hu, C.-M. Yuan, B. Xu, M.-L. Yang, F.-X. Dong and G.-L. Xue, CrystEngComm, 2013, 15, 1460 RSC.
- J. Heine, J. Schmedt auf der Günne and S. Dehnen, J. Am. Chem. Soc., 2011, 133, 10018 CrossRef CAS PubMed; E. C. Constable, G. Zhang, C. E. Housecroft and J. A. Zampese, CrystEngComm, 2011, 13, 6864 RSC; V. N. Dorofeeva, S. V. Kolotilov, M. A. Kiskin, R. A. Polunin, Z. V. Dobrokhotova, O. Cador, S. Golhen, L. Ouahab, I. L. Eremenko and V. M. Novotortsev, Chem. – Eur. J., 2012, 18, 5006 CrossRef PubMed; Y.-Q. Chen, G.-R. Li, Y.-K. Qu, Y.-H. Zhang, K.-H. He, Q. Gao and X.-H. Bu, Cryst. Growth Des., 2013, 13, 901 Search PubMed; C. Liu, Y.-B. Ding, X.-H. Shi, D. Zhang, M.-H. Hu, Y.-G. Yin and D. Li, Cryst. Growth Des., 2009, 9, 1275 Search PubMed; J. Song, B.-C. Wang, H.-M. Hu, L. Gou, Q.-R. Wu, X.-L. Yang, Y.-Q. Shangguan, F.-X. Dong and G.-L. Xue, Inorg. Chim. Acta, 2011, 366, 134 CrossRef PubMed; K.-R. Ma, F. Ma, Y.-L. Zhu, L.-J. Yu, X.-M. Zhao, Y. Yang and W.-H. Duan, Dalton Trans., 2011, 40, 9774 RSC; Y.-Q. Chen, G.-R. Li, Z. Chang, Y.-K. Qu, Y.-H. Zhang and X.-H. Bu, Chem. Sci., 2013, 4, 3678 RSC.
- See for example: N. Li, Q.-E. Zhu, H.-M. Hu, H.-L. Guo, J. Xie, F. Wang, F.-X. Dong, M.-L. Yang and G.-L. Xue, Polyhedron, 2013, 49, 207 CrossRef CAS PubMed; B.-C. Wang, X.-L. Chen, H.-M. Hu, H.-L. Yao and G.-L. Xue, Inorg. Chem. Commun., 2009, 12, 856 CrossRef PubMed; N. Li, H.-L. Guo, H.-M. Hu, J. Song, B. Xu, M.-L. Yang, F.-X. Dong and G.-L. Xue, J. Solid State Chem., 2013, 198, 416 CrossRef PubMed; B.-C. Wang, Q.-R. Wu, H.-M. Hu, X.-L. Chen, Z.-H. Yang, Y.-Q. Shangguan, M.-L. Yang and G.-L. Xue, CrystEngComm, 2010, 12, 485 RSC.
- G. W. V. Cave and C. L. Raston, J. Chem. Soc., Perkin Trans. 1, 2001, 3258 CAS.
- J. Yoshida, S.-I. Nishikiori and H. Yuge, J. Coord. Chem., 2013, 66, 2191 CrossRef CAS.
- S. A. S. Ghozlan and A. Z. A. Hassanien, Tetrahedron, 2002, 58, 9423 CrossRef CAS.
- E. C. Constable, C. E. Housecroft, S. Vujovic and J. A. Zampese, CrystEngComm, 2014, 16, 3494 RSC.
- S. R. Batten, S. M. Neville and D. R. Turner, Coordination Polymers: Design, Analysis and Application, RSC Publishing, Cambridge, 2009 Search PubMed.
- H. D. Flack, Helv. Chim. Acta, 2003, 86, 905 CrossRef CAS.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS.
- V. A. Blatov and A. P. Shevchenko, TOPOS Professional v. 4.0, Samara State University, Russia Search PubMed.
- D.-S. Li, Y.-P. Wu, J. Zhao, J. Zhang and J. Y. Lu, Coord. Chem. Rev., 2014, 261, 1 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic and crystallographic details; Fig. S1–S2 solution 1H NMR and absorption spectra of 1. CCDC 1035825. See DOI: 10.1039/c4ce02347a |
| This journal is © The Royal Society of Chemistry 2015 |