Isostructurality in six celecoxib co-crystals introduced by solvent inclusion†
Abstract
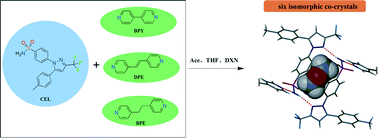
Three co-crystals of celecoxib (CEL) and bipyridine derivatives were synthesized based on the synthon design approach and structurally characterized. The resulting two-component complexes have different unit cell parameters and noticeably different packing patterns. Surprisingly, when different organic solvents are introduced into the binary-host crystal systems, six isostructural three-component crystals are obtained. These isomorphic complexes are formed by CEL with two different sets of bipyridine co-formers containing various solvent molecules. A robust H-bonding framework between CEL dimer molecules is found to tolerate subtle changes in the structures of the co-formers and guest solvent molecules. The physicochemical properties of these isomorphic co-crystals are fully examined and compared with their corresponding non-solvate forms. The desolvation mechanism and relative thermodynamic stabilities are also discussed.


 Please wait while we load your content...
Please wait while we load your content...