Hydrogen bonded-extended lanthanide coordination polymers decorated with 2,3-thiophenedicarboxylate and oxalate: synthesis, structures, and properties†
Abstract
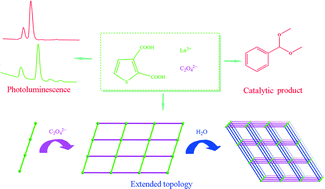
Five new lanthanide coordination polymers, {[Ln2(tdc)2(C2O4)(H2O)4]·(H2O)}n (Ln = Nd (1), Eu (2), Gd (3), Tb (4), Ho (5); H2tdc = 2,3-thiophenedicarboxylic acid), have been synthesized by hydrothermal reactions of H2tdc, sodium oxalate and the corresponding lanthanide nitrate. Crystallographic data show that 1–5 are isomorphous and crystallize in the monoclinic space group C2/c. Each complex contains an oxalate-linked two-dimensional (2D) sql layer structure based on dinuclear [Ln2(tdc)2(H2O)4] building units. Then the 2D layers are further extended by intermolecule hydrogen bonds to form a three-dimensional (3D) supramolecular network with sxb topology. Compounds 2 and 4 display luminescence emission in the visible region, while compounds 1 and 5 exhibit luminescence emission in the near IR region. Dehydrated 1 as a Lewis acid catalyst exhibits high catalytic activity and stability in acetalization reaction under mild conditions. Furthermore, infrared, thermogravimetric analysis, elemental analyses, and powder X-ray diffraction properties of these compounds are also studied.

 Please wait while we load your content...
Please wait while we load your content...