The influence of anion, ligand geometry and stoichiometry on the structure and dimensionality of a series of AgI-bis(cyanobenzyl)piperazine coordination polymers†
Abstract
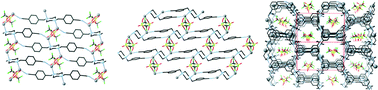
Two new bis-(cyanobenzyl)piperazine ligands have been prepared, and have been used to prepare five Ag(I) coordination polymers which have been structurally characterised. Ligand N,N′-bis((4-cyanophenyl)methyl)piperazine L1 gave two structurally related two-dimensional coordination polymers poly-[Ag2(L1)(CF3SO3)2] 1 and poly-[Ag2(L1)(CF3SO3)2(C3H6O)1.5] 2 when reacted with silver trifluoromethanesulfonate in tetrahydrofuran and acetone, respectively. Compounds 1 and 2 display similar ligand geometries but form different extended networks, as a result of the inclusion of coordinating solvent molecules and the resulting influence on the flexible metal geometry. Reaction of N,N′-bis((3-cyanophenyl)methyl)piperazine L2 with silver ions gave rise to three new structurally characterised species. Complex poly-[Ag2(L2)(C3H6O)2]2(PF6) 3 forms a one-dimensional chain structurally related to 1. Complex poly-[Ag2(L2)(C3H8O)2(CF3SO3)2] 4 formed under similar conditions to 3, except with the inclusion of a coordinating anion which links equivalent chains to those found in 3 into a two-dimensional sheet. Finally, complex poly-[AgL2]CF3SO35 was formed simply by altering the reaction stoichiometry, producing a three-dimensional PtS network containing linear, acentric anion channels. Analysis of the structures reveals that the ligand geometries are largely consistent, leading to the extended structures being more influenced by the nature of the anions and solvent molecules.
- This article is part of the themed collection: International Year of Crystallography Celebration: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...