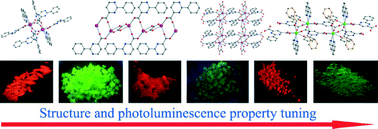
Six novel luminescent lanthanide-2-phenylpyrimidine-carboxylate frameworks, namely, [Ln(ppmc)3(phen)] (Ln = Eu (1), Tb (2)), [Ln(ppmc)2(C2O4)0.5(H2O)] (Ln = Eu (3), Tb (4)), {[Eu(ppmdc)(phen)(C2O4)0.5]·0.5H2O} (5) and [Tb(ppmdc)(benzoate)(phen)] (6) (Hppmc = 2-phenylpyrimidine-4-carboxylic acid, H2ppmdc = 2-phenylpyrimidine-4,6-dicarboxylic acid) have been synthesized. The X-ray structure analyses reveal that all of the compounds contain carboxylate-bridged dimer units; however, coordination environments of Ln3+ ions, bridging modes of carboxylate, and the linkage between them are different. In the isostructural compounds 1 and 2, extensive π–π stacking exists between the anti–anti carboxylate bridged dimer. However, syn–anti carboxylate bridges are found in compounds 3 and 4 in which oxalate connects the dimers to form 1D chains. Compound 5 has a 2D structure connected by both μ4-ppmdc2− and oxalate in which the carboxylates adopt both anti–anti and chelate-bridging modes. Compound 6 featuring 1D chains also contains anti–anti carboxylate-bridged dimers that are linked by the μ3-ppmdc2− ligand. All six compounds show characteristic red or green emission attributed to Eu3+ and Tb3+. The luminescence lifetime and quantum yield are presented and discussed in detail. The use of different ligands and reaction conditions has led to distinct coordination environments of lanthanide ions and the aggregation of dimeric units, which are considered as the dominating factors for the resulting photoluminescence behaviour of solid samples.