Open Access Article
Open Access ArticleStructural transformations in crystals induced by radiation and pressure. Part 1. How pressure influences the intramolecular photochemical reactions in crystals†
Julia
Bąkowicz
* and
Ilona
Turowska-Tyrk
Faculty of Chemistry, Wrocław University of Technology, Wybrzeże Wyspiańskiego 27, 50-370 Wrocław, Poland. E-mail: julia.bakowicz@pwr.wroc.pl; Fax: +48 71 320 33 64
First published on 21st May 2014
Abstract
The main aim of the studies in this paper is to gain knowledge on the path of structural transformations resulting from the photocyclization in 2-tert-butylphenylphenylmethanone crystals at low and high pressures and also to carry out a comparative analysis of the results at different pressures. This work is the first example of these types of studies in scientific literature. We determined the structure of: pure reactant crystals, i.e. only containing reactant molecules, at 0.1 MPa, 0.55 GPa, 1.27 GPa and 1.50 GPa; pure product crystals, i.e. only containing product molecules, at 0.55 GPa and 1.27 GPa; and partly reacted crystals (ten structures), i.e. containing both reactant and product molecules in various proportions to each other, at 0.1 MPa (two structures), 0.55 GPa (three structures), 1.27 GPa (three structures) and 1.50 GPa (two structures). The studies have shown that (a) there is no phase transition when an increase in pressure is imposed onto the crystals, (b) the unit cell parameters change non-linearly with an increase in pressure, (c) the photochemical reaction was conducted in a homogeneous manner in the examined crystals, (d) the reaction brings about different modes of changes in the unit cell parameters at different pressures (e) the reaction proceeds faster at the end rather than at the beginning regardless of the pressure imposed onto the crystals.
Introduction
Structural changes induced in crystals by photochemical reactions at ambient pressure
Photochemical reactions in crystals are of interest to many scientists. In recent years, papers on monitoring structural changes in crystals brought about by photochemical reactions have started to appear. These publications include both inter- and intramolecular reactions: mainly [2 + 2] and [4 + 4] photodimerizations,1–6 a Norrish–Yang reaction (which is based on the formation of a four-membered ring (Scheme 1a)),7–10 and E → Z or Z → E photoisomerizations.11–14 The careful monitoring of the structures for many stages of crystal transformations revealed that: (a) unreacted molecules do not assume a constant position in the crystals, (b) in the case of [2 + 2] and [4 + 4] photodimerizations, the unreacted molecules come closer to each other and the distance between the reactive atoms decreases linearly with the progression of crystal transformation; the observed relationship was explained by the stress imposed by product molecules onto reactant molecules, (c) for most of the intramolecular reactions, the distance between the reactive atoms is constant during a long period of crystal transformation, (d) the orientations of the molecular fragments are changed as the transformation progresses; the biggest changes are ca. 20°, (e) the rate of the photochemical reactions depends on the concentration of the reactant molecules in the crystals and (f) the photochemical reactions bring about changes in the unit cell parameters, which is a result of changes in the crystal structures as the reactions progress.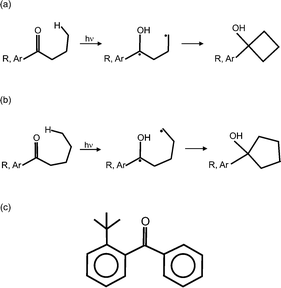 | ||
| Scheme 1 The photocyclization leading to (a) a four and (b) a five-membered ring, with (c) the structure of the studied compound. | ||
Chemical reactions in crystals at high pressure
High-pressure techniques are important and useful tools in materials science. High pressure can induce various changes in crystal and molecular structures, for instance: shortening the distances between atoms and molecules, changing the molecular orientation, decreasing the cell volume and the volume of reaction cavities and also decreasing the values of atomic displacement parameters.15,16 High pressure can also bring about phase transitions and chemical reactions.Studies of pressure-induced chemical reactions (without photo-induction) have been carried out for two decades. Until now, chemical reactions in crystals at high pressure have been reported for small molecular systems17 like nitrogen, carbon monoxide, carbon dioxide and nitrous oxide, for simple unsaturated compounds, in particular for hydrocarbons17–26 such as acetylene, ethylene, propylene, butadiene, for cyano derivatives and aromatics compounds17,18,27–34 like benzene and styrene. At high pressure, most of them undergo polymerization, addition and amorphization.
Some of the reported transformations were induced not only by high pressure, but simultaneously by electromagnetic radiation. In many cases the use of radiation at a high pressure enables different compounds with different properties to be obtained. Such a situation is observed for the polymerization of butadiene.17,18,35–37
Most chemical reactions at high pressure are studied using spectroscopic methods, usually IR and Raman spectroscopy, and very rarely, the final products and their structures are characterized by X-ray diffraction methods, usually powder diffraction.
Here, we present the results of an X-ray structure analysis on how pressure modifies structural changes brought about by the photochemical reaction in a crystal. This is the first example of such studies in scientific literature. The main aim of this research was accomplished by: (a) monitoring the deformations of the cell constants with the progression of the photochemical reaction at different pressures, (b) monitoring the deformations of the cell constants with an increase in pressure, i.e. without photo-induction, (c) monitoring the content of photoproduct molecules in crystals at different pressures, (d) analysing the changes in shape and geometry of the molecules in the crystals, (e) analysing the changes in molecular orientation. In order to achieve these goals, we determined pure reactant crystal structures, pure product crystal structures and also crystal structures of partly reacted crystals, i.e. containing both reactant and product molecules, at ambient and high pressures. The compound studied was 2-tert-butylphenylphenylmethanone.
Experimental
The studied compound was purchased from Sigma-Aldrich and recrystallized from a methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol
ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture (vol. 16
water mixture (vol. 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
The experiments were carried out at ambient and high pressure and in the dark. In the high-pressure diffraction experiments, a high-pressure Boehler–Almax diamond anvil cell (DAC) was used.38 A single crystal of the studied compound, quartz and a small ruby chip were glued to a culet surface of the diamond anvil, covered by an inconel gasket (thickness: 0.3 mm, hole diameter: 0.35 mm), and filled with a glycerin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture (vol. 3
water mixture (vol. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) or hydrostatic glycerin liquid. The quartz and ruby crystals acted as pressure sensors. The high pressure values in the DAC were estimated by the ruby fluorescence method39,40 and/or by the unit cell parameters of quartz.41 The DAC was mounted on a diffractometer and aligned using the gasket-shadow centering procedure.42 The sets of collected reflections contained data coming from the two diamonds, quartz, ruby and the studied compound. Nevertheless, it was possible to remove the reflections coming from both of the diamonds and to group the remaining sets into three separate parts: those coming from the quartz, those from the ruby and those from the studied compound. We used five different crystals of the studied compound. Four of them were closed in the DAC and one was mounted on a glass pin and measured at ambient pressure (0.1 MPa). The values of high pressure were as follows: 0.55, 0.90, 1.27 and 1.50 GPa. The CrysAlisPro program suite43 was used for data collection, UB matrices determination and data reduction. Additional corrections of the reflection intensities, associated with DAC absorption, were unnecessary.
2) or hydrostatic glycerin liquid. The quartz and ruby crystals acted as pressure sensors. The high pressure values in the DAC were estimated by the ruby fluorescence method39,40 and/or by the unit cell parameters of quartz.41 The DAC was mounted on a diffractometer and aligned using the gasket-shadow centering procedure.42 The sets of collected reflections contained data coming from the two diamonds, quartz, ruby and the studied compound. Nevertheless, it was possible to remove the reflections coming from both of the diamonds and to group the remaining sets into three separate parts: those coming from the quartz, those from the ruby and those from the studied compound. We used five different crystals of the studied compound. Four of them were closed in the DAC and one was mounted on a glass pin and measured at ambient pressure (0.1 MPa). The values of high pressure were as follows: 0.55, 0.90, 1.27 and 1.50 GPa. The CrysAlisPro program suite43 was used for data collection, UB matrices determination and data reduction. Additional corrections of the reflection intensities, associated with DAC absorption, were unnecessary.
We induced the photochemical reaction using UV radiation. The crystals were irradiated in steps using an Hg 100 W lamp equipped with a water filter and a WG-320 glass filter. The WG-320 filter blocked shorter and transmitted longer wavelengths: 0% transmittance for λ < 300 nm, ca. 95% transmittance for λ = 350 nm and 100% transmittance for λ > 365 nm. Such wavelengths helped us to conduct the reaction homogenously.44,45 The intensity of the UV beam was 800 mW cm−2. The irradiation times were: 0, 10, 20 and 40 min in total at 0.1 MPa; 0, 10, 30, 60 and 120 min in total at 0.55 GPa; 0, 15, 30, 60, 90, 120 and 180 min in total at 0.90 GPa; 0, 30, 90, 180 and 300 min in total at 1.27 GPa; 0, 40, 80 and 150 min in total at 1.50 GPa. The structure of the non-irradiated crystal of the studied compound at ambient pressure has been published previously.46 We redetermined it to ensure that all of the data were from the same sample. The structures were solved by means of SHELXS97 and refined using SHELXL97.47 The crystal data obtained at 0.90(2) GPa, at ambient pressure for 40 min UV irradiation and at 1.50 GPa for 150 min UV irradiation were not of satisfactory quality and were omitted in the paper. The final refined values of the site occupation factor revealed the following content of the product: 0, 5.9(5), and 10.6(6)% for the crystal at 0.1 MPa; 0, 7.3(6), 15.4(9), 44.0(13), and 100% for the crystal at 0.55 GPa; 0, 7.8(6), 20.5(10), 51.9(16), and 100% for the crystal at 1.27 GPa and 0, 11.2(9) and 23.8(12)% for the crystal at 1.50 GPa. Owing to a reactant–product disorder, the following weak restraints from SHELXL9747 were applied: DFIX, DANG and SIMU. The target values of the bond lengths and valence angles were taken from the structures of the pure reactant and pure product crystals.
Some non-hydrogen atoms of the major component were refined anisotropically, but all of the atoms of the minor component were treated isotropically. Hydrogen atoms were positioned geometrically with Uiso = 1.2 Ueq for benzene rings and Uiso = 1.5 Ueq for methyl groups. The hydrogen atom from the hydroxyl group in the product molecule was omitted in the case of all partly reacted crystals. For the pure product crystals, it was located in difference Fourier maps.
Selected experimental data are presented in Table 1 for the structures at 0.55 GPa. Data for all structures are given in the cif files.
| 0.0% P | 7.3% P | 15.4% P | 44.0% P | 100.0% P | |
|---|---|---|---|---|---|
| Chemical formula | C17H18O | C17H18O | C17H18O | C17H18O | C17H18O |
| Formula weight | 238.31 | 238.31 | 238.31 | 238.31 | 238.31 |
| Crystal system | Orthorhombic | Orthorhombic | Orthorhombic | Orthorhombic | Orthorhombic |
| Space group | Pbca | Pbca | Pbca | Pbca | Pbca |
| a/Å | 13.4655(10) | 13.4910(9) | 13.5452(14) | 13.649(3) | 13.8701(12) |
| b/Å | 12.5668(9) | 12.5526(9) | 12.5231(13) | 12.434(2) | 12.3384(12) |
| c/Å | 15.7267(10) | 15.7300(10) | 15.7348(15) | 15.727(2) | 15.6455(14) |
| V/Å3 | 2661.2(3) | 2663.8(3) | 2669.1(5) | 2669.1(8) | 2677.5(4) |
| Z | 8 | 8 | 8 | 8 | 8 |
| D x/Mg m−3 | 1.190 | 1.188 | 1.186 | 1.186 | 1.182 |
| μ/mm−1 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| T/K | 299(2) | 299(2) | 299(2) | 299(2) | 299(2) |
| Reflections collected | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 156 156 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 174 174 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 127 127 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 278 278 |
| Reflections independent | 1704 | 1707 | 1711 | 1712 | 1718 |
| Reflections observed | 957 | 942 | 866 | 763 | 931 |
| R int | 0.083 | 0.085 | 0.097 | 0.121 | 0.092 |
| R, wR (F2 > 2σ(F2)), S | 0.057, 0.119, 1.03 | 0.068, 0.142, 1.04 | 0.076, 0.168, 1.05 | 0.108, 0.260, 1.04 | 0.061, 0.126, 1.06 |
| Δρmax, Δρmin/e Å−3 | 0.16, −0.18 | 0.23, −0.22 | 0.20, −0.21 | 0.29, −0.20 | 0.19, −0.17 |
In order to check whether the photochemical reaction stops after the removal of the source of UV radiation, an additional data collection was carried out 24 h after the data collection for one of the partly reacted crystals. The content of the product, determined during the structure refinement, was statistically the same for both data sets. This also showed that the photochemical reaction was not influenced by X-rays.
Results and discussion
Under satisfactory conditions 2-tert-butylphenylphenylmethanone, the studied compound, can undergo a photocyclization reaction in crystals (Scheme 1b and c). In this reaction, a δ-H atom is transferred to a carbonyl group and as a result a 1,5-diradical is formed which afterwards can lead to the formation of a five-membered ring.Fig. 1a shows the DAC used in the high pressure studies and a typical collection of crystals placed in a metal gasket in the DAC, namely a crystal of the studied compound and additionally ruby and quartz crystals used as pressure sensors. Fig. 1b presents the structures of the molecules present in a pure reactant crystal, in one of the partly reacted crystals and in a pure product crystal at high pressure. It is noticeable that the shapes of the reactant and product molecules are very similar and also that the phenyl ring moved more in the reaction than the 2-tert-butylphenyl moiety did.
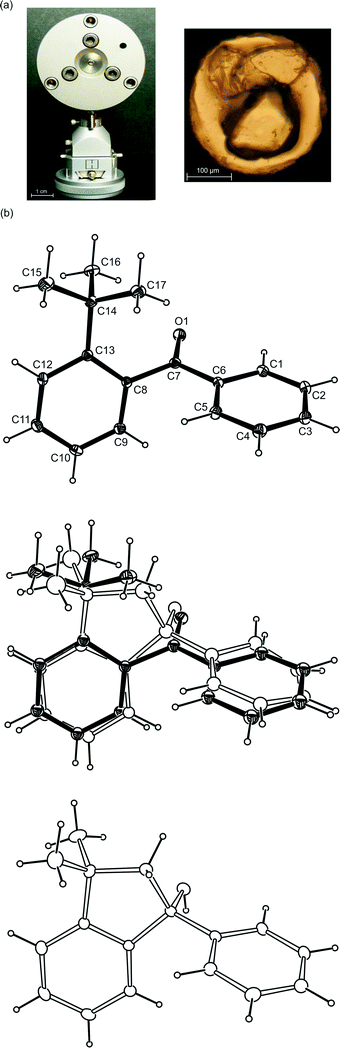 | ||
| Fig. 1 (a) Diamond anvil cell (DAC) mounted on a goniometer head and crystals placed in the hole of a metal gasket at 0.55 GPa: ruby, quartz and the studied compound. (b) ORTEP48 view of the molecules in the pure reactant (upper), partly reacted (middle) and pure product (lower) crystals at 0.55 GPa. | ||
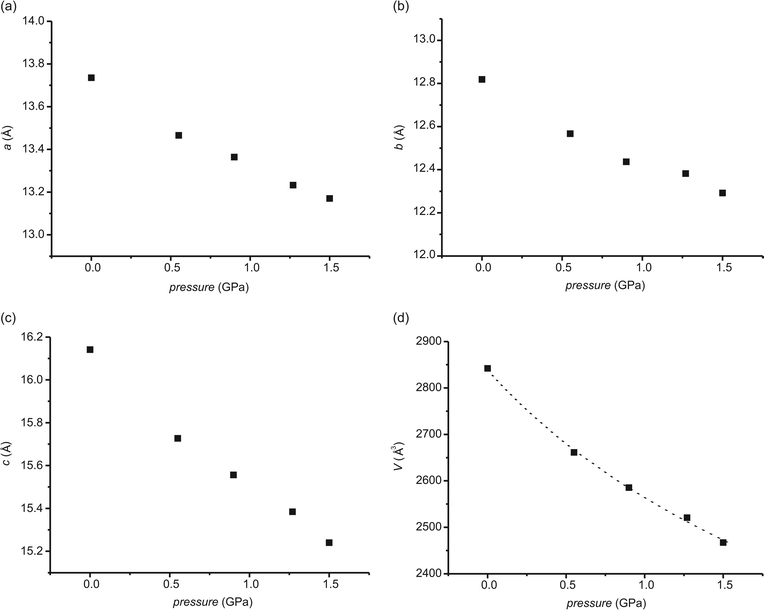
Structural changes brought about by pressure, without photo-induction
Fig. 2 shows the variations in the unit cell parameters and the unit cell volume with the pressure imposed on the crystals. The changes in the a, b and c parameters en route from the ambient pressure to the pressure of 1.50 GPa are −0.572, −0.517 and −0.907 Å, which is equivalent to 4.2, 4.0 and 5.6%, respectively. The decrease in the percentage of the unit cell parameters along the a and b axis is very similar. A rather large change in the cell volume, 13.2%, is not unusual for molecular compounds.49,50 The changes in the cell parameters are not linear and are bigger at the beginning of the pressure imposition. This is understandable since intermolecular distances are longer at lower pressures and it is easier to shorten them. The pressure–volume data are in agreement with the Birch–Murnaghan equation of state:51| P = 3/2K0[(V0/V)7/3–(V0/V)5/3]{1 − 3/4(4 − K′0)[(V0/V)2/3 − 1]}, | (1) |
V 0 – the cell volume at ambient pressure,
K 0 – the ambient-pressure bulk modulus (incompressibility) and
K ′0 – the pressure derivative of incompressibility.
The least-squares fit of the pressure–volume (compression) data for the studied compound to the Birch–Murnaghan equation of state gave K0 = 7.3 GPa and K′0 = 5.4 GPa.
The intermolecular distances were also visualized by the Hirshfeld surfaces52,53 in Fig. 3. As can be seen, big changes take place near the reaction centre.
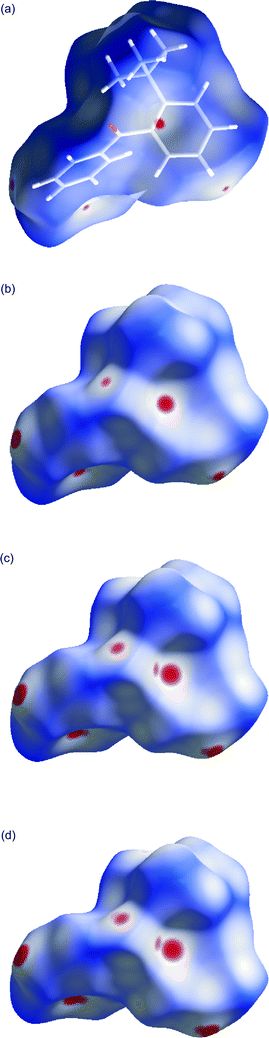 | ||
| Fig. 3 The Hirshfeld surfaces for the crystals studied at (a) 0.1 MPa, (b) 0.55 GPa, (c) 1.27 GPa and (d) 1.50 GPa, calculated as dnorm by the CrystalExplorer program.54 The red colour denotes the intermolecular contacts that are shorter than the sum of the van der Waals radii of interacting atoms, white is for the van der Waals contacts and blue is for the longer contacts. | ||
The variations in the unit cell parameters and the cell volume are smooth, which indicates that no phase transition takes place in the pressure range from ambient to 1.50 GPa. A smooth relationship is also observed between the values of the free space in the crystals and the values of the pressure, which is shown in Fig. 4a. This also indicates that there is no phase transition brought about by a change in pressure. From Fig. 4b, showing the free space in the unit cell, it can be seen how much free space decreases in the proximity of the molecules, especially near the phenyl and 2-tert-butylphenyl fragments, with an increase in pressure.
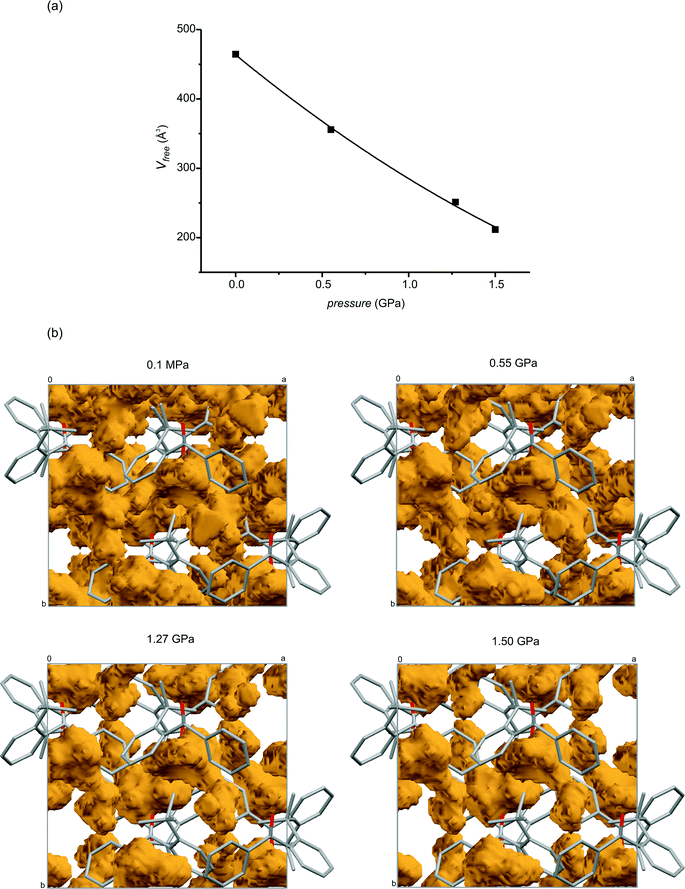 | ||
| Fig. 4 (a) Relationship between the free space in the unit cell and the pressure. (b) The free space for the crystals studied at 0.1 MPa, 0.55 GPa, 1.27 GPa and 1.50 GPa. The rolling ball method from the Mercury program55 was applied; the ball radius and grid were 0.6 and 0.2 Å, respectively. | ||
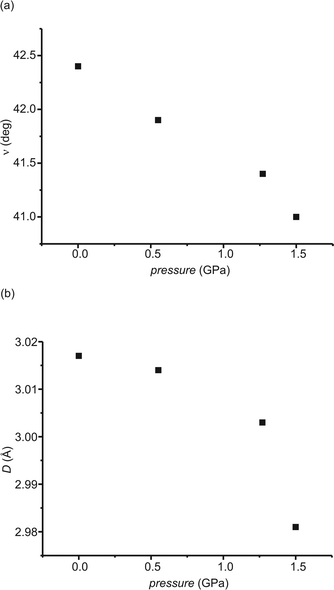
The orientation of the molecules in the crystals does not change significantly en route from 0.1 MPa to 1.50 GPa. The biggest change observed is for the benzene ring, 1.4°, but the change in orientation proceeds in a smooth manner (shown in Fig. 5a).
High pressure influenced not only the intermolecular distances but also the intramolecular non-bonding contacts: for instance, the distance between the carbon atoms which take part in the formation of a five-membered ring in the photochemical reaction, D in Fig. 5b. As can be seen, D is almost constant for lower values of pressure and changes only at high values. High pressure is not able to significantly change the dihedral angle between the planes of both of the benzene rings in the molecules. This angle decreases only by 1.1° en route from 0.1 MPa to 1.50 GPa, but nevertheless even this decrease proceeds in a smooth manner.
Photo-induced structural changes at different pressures
| P = 1 − exp[−(kt)n], | (2) |
P – the fraction of a product in a crystal,
k – the rate constant of a photochemical reaction,
t – the time of irradiation of a crystal and
n – the Avrami exponent describing the dimensionality of growth of nuclei.
The values of n equal to 1, 2, 3 or 4 prove that the formation of a product in crystals is homogenous, linear, two-dimensional or three-dimensional, respectively. Intermediate values indicate a hybrid mechanism.
In the case of the crystals studied in this work, the values of n are: 0.9, 1.1(3), 1.2(2) and 1.2 for 0.1 MPa, 0.55 GPa, 1.27 GPa and 1.50 GPa, respectively. They indicate that the photochemical reaction in the examined crystals was mainly conducted in a homogenous manner.
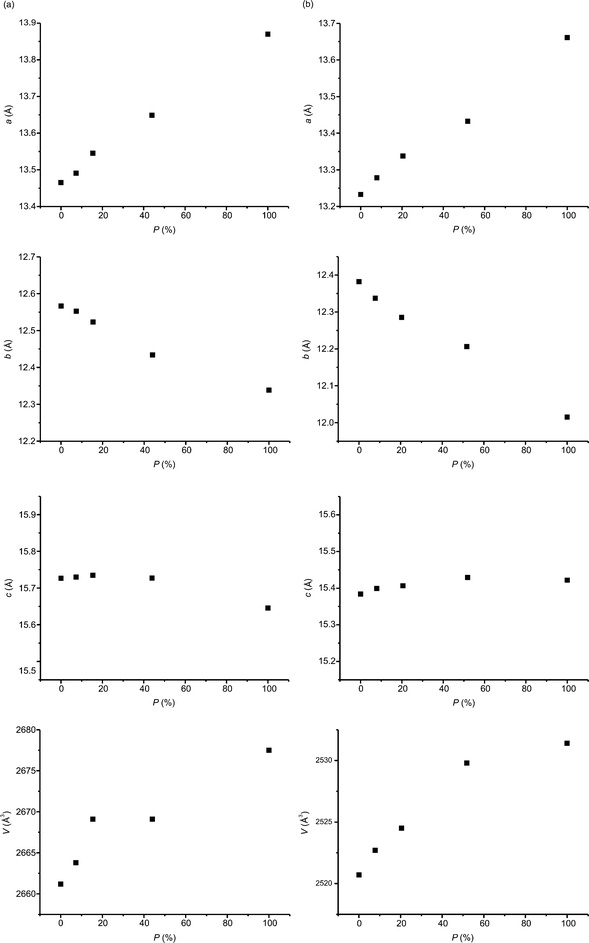
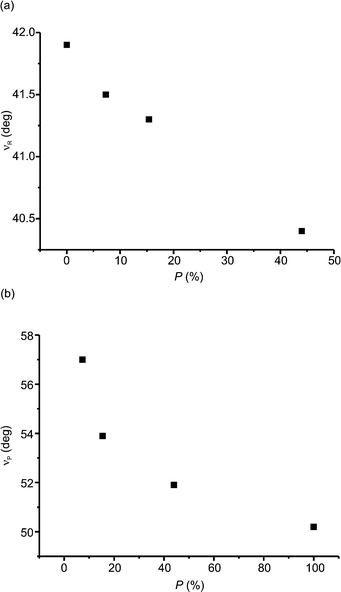
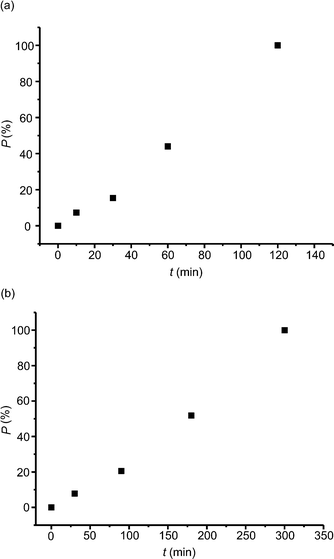
Fig. 8 reveals the changes in the content of the product molecules in the crystal along with the time of irradiation of the crystal at pressures of 0.55 GPa and 1.27 GPa. As can be seen, the relationships are neither exponential nor linear, which indicates that the photochemical reaction is neither first nor zero order. For both pressures the photochemical reaction proceeds faster at the end rather than at the beginning. In the case of the Norrish–Yang reaction conducted at ambient pressure in α-methylbenzylamine salt with 1-(4-carboxybenzoyl)-1-methyladamantane,7 the salt of 6,6-diethyl-5-oxo-5,6,7,8-tetrahydronaphthalene-2-carboxylic acid with (1S)-1-(4-methylphenyl)-ethylamine9 and methyl 2-{[4-(2,4,6-triisopropylbenzoyl)benzoyl]amino}-3-phenylpropanoate (enantiomeric form)10 we observed exponential relationships. However, for methyl 2-{[4-(2,4,6-triisopropylbenzoyl)benzoyl]amino}-3-phenylpropanoate (racemic form)10 the relationship between the content of the product and the time of irradiation has a character similar to that observed for the compound studied in this work, i.e. the reaction speeds up at the end. Now, we can generalize these observations in the following way. For all reactions characterized by exponential relationships, the distance between the reactive atoms in reactant molecules, D, is constant during the whole or almost the whole crystal phototransformation.7,9,10 This is not the case for the compounds where an increase in the speed of the reaction rate is observed. For them, D is not constant but decreases.10 (In general, smaller distances between reactive atoms are expected to increase the rates of photochemical reactions.) The decrease in D is also observed for the compound studied in this paper, namely from 3.014(5) Å (0% reaction progress) to 2.942(18) Å (44.0% reaction progress) at a pressure of 0.55 GPa and from 3.004(5) Å (0% reaction progress) to 2.88(3) Å (51.9% reaction progress) at 1.27 GPa.
Conclusions
The analysis of intra- and intermolecular geometrical parameters for many stages of the photocyclization of 2-tert-butylphenylphenylmethanone provided information on the changes in a reaction centre, molecules and crystals and information on the influence of external factors, namely pressure. The studies showed that (a) the photochemical reaction carried out for the compound investigated brings about different modes of changes in the unit cell parameters at different pressures, (b) the reaction proceeds faster at the end than at the beginning, regardless of the pressure imposed onto the crystals, (c) the reaction was conducted in a homogeneous manner (d) there is no phase transition when an increase in pressure is imposed onto the crystals.Further research on other compounds and other types of photochemical reactions (intra- and intermolecular) will enable a better understanding of the influence of external factors, namely pressure, on the reactions in crystals and the paths of transformations of crystals.
Acknowledgements
The work was carried out with the grant 2011/01/D/ST5/02834 financed by the National Science Centre (Poland) and the fellowship was co-financed by the European Union with the European Social Fund (Wrocław University of Technology, Poland). The authors thank Prof. A. Katrusiak for a discussion concerning data collection strategy at high-pressure and Dr E. Zienkiewicz for help with the Birch–Murnaghan fit.References
- I. Turowska-Tyrk, Chem. – Eur. J., 2001, 7, 3401–3405 CrossRef CAS.
- I. Turowska-Tyrk, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 670–675 Search PubMed.
- I. Turowska-Tyrk and E. Trzop, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 779–786 Search PubMed.
- S. Ohba and Y. Ito, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 149–155 Search PubMed.
- M. A. Fernandes and D. C. Levendis, Acta Crystallogr., Sect. B: Struct. Sci., 2004, 60, 315–324 CAS.
- J. B. Benedict and P. Coppens, J. Phys. Chem. A, 2009, 113, 3116–3120 CrossRef CAS PubMed.
- I. Turowska-Tyrk, E. Trzop, J. R. Scheffer and S. Chen, Acta Crystallogr., Sect. B: Struct. Sci., 2006, 62, 128–134 Search PubMed.
- I. Turowska-Tyrk, I. Łabęcka, J. R. Scheffer and W. Xia, Pol. J. Chem., 2007, 81, 813–824 CAS.
- I. Turowska-Tyrk, J. Bąkowicz and J. R. Scheffer, Acta Crystallogr., Sect. B: Struct. Sci., 2007, 63, 933–940 CAS.
- J. Bąkowicz, J. Skarżewski and I. Turowska-Tyrk, CrystEngComm, 2011, 13, 4332–4338 RSC.
- S. Zheng, M. Messerschmidt and P. Coppens, Acta Crystallogr., Sect. B: Struct. Sci., 2007, 63, 644–649 CAS.
- S. Zheng, M. Messerschmidt and P. Coppens, Chem. Commun., 2007, 2735–2737 RSC.
- S. Zheng, C. M. L. Vande Velde, M. Messerschmidt, A. Volkov, M. Gembicky and P. Coppens, Chem. – Eur. J., 2008, 14, 706–713 CrossRef CAS PubMed.
- P. Coppens, S. Zheng and M. Gembicky, Z. Kristallogr., 2008, 223, 265–271 CrossRef CAS.
- E. V. Boldyreva, in Reactivity of Solids. Past, Present, Future, ed. V. Boldyrev, IUPAC Series, 1996, pp. 141–184 Search PubMed.
- E. V. Boldyreva, Solid State Ionics, 1997, 101–103, 843–849 CrossRef CAS.
- V. Schettino and R. Bini, Phys. Chem. Chem. Phys., 2003, 5, 1951–1965 RSC.
- R. Bini, Acc. Chem. Res., 2004, 37, 95–101 CrossRef CAS PubMed.
- K. Aoki, S. Usuba, M. Yoshida, Y. Kakudate, K. Tanaka and S. Fujiwara, J. Chem. Phys., 1988, 89, 529–534 CrossRef CAS PubMed.
- D. Chelazzi, M. Ceppatelli, M. Santoro, R. Bini and V. Schettino, Nat. Mater., 2004, 3, 470–475 CrossRef CAS PubMed.
- D. Chelazzi, M. Ceppatelli, M. Santoro, R. Bini and V. Schettino, J. Phys. Chem. B, 2005, 109, 21658–21663 CrossRef CAS PubMed.
- M. Santoro, L. Ciabini, R. Bini and V. Schettino, J. Raman Spectrosc., 2003, 34, 557–566 CrossRef CAS.
- M. Ceppatelli, M. Santoro, R. Bini and V. Schettino, J. Chem. Phys., 2000, 113, 5991–6000 CrossRef CAS PubMed.
- M. Sakashita, H. Yamawaki and K. Aoki, J. Phys. Chem., 1996, 100, 9943–9947 CrossRef CAS.
- C. C. Trout and J. V. Badding, J. Phys. Chem. A, 2000, 104, 8142–8145 CrossRef CAS.
- M. Mugnai, M. Pagliai, G. Cardini and V. Schettino, J. Chem. Theory Comput., 2008, 4, 646–651 CrossRef CAS.
- J. M. Besson, M. M. Thiery and P. Pruzan in Molecular Systems Under High Pressure, ed. R. Pucci and G. Piccitto, Elsevier, Amsterdam, 1991, p. 341 Search PubMed.
- L. Ciabini, M. Santoro, R. Bini and V. Schettino, J. Chem. Phys., 2002, 116, 2928–2935 CrossRef CAS PubMed.
- P. Pruzan, J. C. Chervin, M. M. Thiery, J. P. Itie and J. M. Besson, J. Chem. Phys., 1990, 92, 6910–6915 CrossRef CAS PubMed.
- L. Ciabini, M. Santoro, F. A. Gorelli, R. Bini, V. Schettino and S. Raugei, Nat. Mater., 2007, 6, 39–43 CrossRef CAS PubMed.
- L. Ciabini, M. Santoro, R. Bini and V. Schettino, Phys. Rev. Lett., 2002, 88, 085505 CrossRef.
- M. Ceppatelli, M. Santoro, R. Bini and V. Schettino, J. Chem. Phys., 2003, 118, 1499–1506 CrossRef CAS PubMed.
- M. Santoro, M. Ceppatelli, R. Bini and V. Schettino, J. Chem. Phys., 2003, 118, 8321–8325 CrossRef CAS PubMed.
- Ph. Pruzan, J. C. Chervin and J. P. Forgerit, J. Chem. Phys., 1992, 96, 761–767 CrossRef CAS PubMed.
- V. Schettino, R. Bini, G. Cardini, M. Ceppatelli, M. Citroni and M. Pagliai, Il Nuovo Cimento B, 2008, 123, 1399–1414 Search PubMed.
- M. Citroni, M. Ceppatelli, R. Bini and V. Schettino, Science, 2002, 295(5562), 2058–2060 CrossRef CAS PubMed.
- M. Citroni, M. Ceppatelli, R. Bini and V. Schettino, J. Chem. Phys., 2003, 118, 1815–1820 CrossRef CAS PubMed.
- R. Boehler, Rev. Sci. Instrum., 2006, 77, 115103 CrossRef PubMed.
- G. J. Piermarini, S. Block, J. D. Barnett and R. A. Forman, J. Appl. Phys., 1975, 46, 2774–2780 CrossRef CAS PubMed.
- H. K. Mao, J. Xu and P. M. Bell, J. Geophys. Res., 1986, 91, 4673–4676 CrossRef CAS.
- R. J. Angel, D. R. Allan, R. Miletich and W. Finger, J. Appl. Crystallogr., 1997, 30, 461–466 CrossRef CAS.
- A. Budzianowski and A. Katrusiak in High-Pressure Crystallography, ed. A. Katrusiak and P. F. McMillan, Kluwer Academic Publishers, Dordrecht Boston London, 2004, pp. 101–112 Search PubMed.
- Oxford Diffraction, CrysAlis CCD and CrysAlis RED, Oxford Diffraction Ltd., Abingdon, England, 2006 Search PubMed.
- V. Enkelman, G. Wegner, K. Novak and K. B. Wagener, J. Am. Chem. Soc., 1993, 115, 10390–10391 CrossRef.
- K. Novak, V. Enkelmann, G. Wegner and K. B. Wagener, Angew. Chem., Int. Ed. Engl., 1993, 32, 1614–1616 CrossRef.
- P. J. Wagner, B. P. Giri, J. C. Scaiano, D. L. Ward, E. Gabe and F. L. Lee, J. Am. Chem. Soc., 1985, 107, 5483–5490 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 1997, 30, 565 CrossRef CAS.
- A. Budzianowski, A. Olejniczak and A. Katrusiak, Acta Crystallogr., Sect. B: Struct. Sci., 2006, 62, 1078–1089 CAS.
- E. V. Boldyreva, T. N. Drebushchak, T. P. Shakhtshneider, H. Sowa, H. Ahsbahs, S. V. Goryainow, S. N. Ivashevskaya, E. N. Kolesnik, V. A. Drebushchak and E. B. Burgina, ARKIVOC XII, 2004, pp. 128–155 Search PubMed.
- F. Birch, Phys. Rev., 1947, 71, 809–824 CrossRef CAS.
- J. Bernstein, Cryst. Growth Des., 2011, 11, 632–650 CAS.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 RSC.
- S. K. Wolff, D. J. Grimwood, J. J. McKinnon, M. J. Turner, D. Jayatilaka and M. A. Spackman, CrystalExplorer (Version 3.0), University of Western Australia, 2012 Search PubMed.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS.
- M. Avrami, J. Chem. Phys., 1939, 7, 1103–1112 CrossRef CAS PubMed.
- W. A. Johnson and R. F. Mehl, Trans. AIME, 1939, 135, 416–458 Search PubMed.
- A. N. Kolmogorov, Izv. Akad. Nauk SSSR Ser. Mat., 1937, 1, 355–359 Search PubMed.
- M. Bertmer, R. C. Nieuwendaal, A. B. Barnes and S. E. Hayes, J. Phys. Chem. B, 2006, 110, 6270–6273 CrossRef CAS PubMed.
- A. G. Jarvis, H. A. Sparkes, S. E. Tallentire, L. E. Hatcher, M. R. Warren, P. R. Raithby, D. R. Allan, A. C. Whitwood, M. C. R. Cockett, S. B. Duckett, J. L. Clark and I. J. S. Fairlamb, CrystEngComm, 2012, 14, 5564–5571 RSC.
- J. Kohout, J. Mater. Sci., 2008, 43, 1334–1339 CrossRef CAS PubMed.
- J. Bąkowicz, J. Olejarz and I. Turowska-Tyrk, J. Photochem. Photobiol., A, 2014, 273, 34–42 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 986674–986689. See DOI: 10.1039/c4ce00320a |
| This journal is © The Royal Society of Chemistry 2014 |