Precision measurement of the growth rate and mechanism of ibuprofen {001} and {011} as a function of crystallization environment†
Abstract
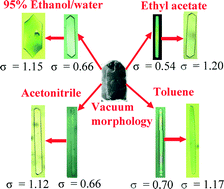
The crystallisation of ibuprofen under diffusion-limited conditions measured as a function of supersaturation, solvent (95% ethanol/water, ethyl acetate, acetonitrile and toluene) and reactor scale is examined. Measurement of solubility as a function of temperature reveals less than ideal behaviour, consistent with strong solute–solute interactions, particularly in the case of acetonitrile. The crystal growth rates of the {001} and {011} faces of spontaneously nucleated crystals are precisely measured in situ using optical microscopy revealing that their respective growth rates increase with increasing supersaturation to different extents, depending on the solvent type, with concomitant impact on the crystal habit. The measured growth rates, as a function of supersaturation, are generally higher at the 15 mL than the 0.5 mL scale size. Analysis of the growth rates versus supersaturation is consistent with a 2-D surface nucleation (Birth and Spread) model for both faces and at both scale sizes. The growth rates of the {001} and {011} faces exhibit much less growth rate dispersion when compared to literature data for a stirred batch crystallizer. The data are rationalised by examining the surface chemistry of the growing faces revealing, e.g. that polar protic solvents inhibit the growth rate of faces containing available binding sites for hydrogen bond formation, such as carboxylic acid groups.

 Please wait while we load your content...
Please wait while we load your content...