Ligand geometry-directed assembly of seven entangled coordination polymers†
Abstract
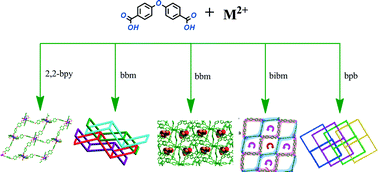
Seven new complexes [Cd(oba)(2,2′-bipy)]2 (1), [Co(oba)(bbm)]·1.5H2O (2), [Mn3(oba)3(bbm)(H2O)2]·DMF·2H2O (3), [Mn2(bibm)2(oba)2] (4), [Co(oba)(bibm)]2·H2O (5), [Co5(bpb)3(oba)4(Hoba)2(H2O)2] (6) and [Ni2(bpb)2(oba)2(DMF)(H2O)]2·5H2O (7) (H2oba = 4,4′-oxydibenzoic acid, bbm = 1,4-di(1H-imidazol-1-yl)benzene, bibm = 4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl, bpb = 1,4-di(pyridin-4-yl)benzene, 2,2′-bipy = 2,2′-bipyridine) have been synthesized and characterized by elemental analysis, IR spectroscopy, thermogravimetric analysis, X-ray powder diffraction and X-ray single crystal diffraction. Compound 1 displays a 3-fold polythreaded 2D network, while 2 has sql layers and these layers are further stacked in a 2D → 3D polycatenation model. Compound 3 shows a 3D porous net with {421·54·63} topology with 1D channels that are filled by DMF and water molecules while 4 possesses a 2D (4,4) layer structure composed of [Mn2(bibm)2(oba)2] units. Compound 5 exhibits a five-fold interpenetrating 3D framework while 6 displays a 2D → 3D polycatenation motif. Compound 7 has a three-fold interpenetrating 3D framework. These results provide some insight into the ligand geometry-driven assembly of entangled coordination polymers. Thermal stability and photoluminescence properties of 1–7 were studied. The optical and photocatalytic properties of 3 and 6 were also investigated.
- This article is part of the themed collection: International Year of Crystallography Celebration: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...