Abstract
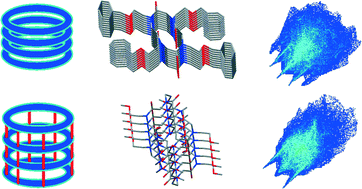
The solid state assembly of free and metal coordinated cyclic α-peptoids has been examined with the aim to find common underlying features and to direct the design of new functional biomimetic materials with desired properties in terms of molecular recognition, drug delivery and catalysis. The lack of the amide proton prevents the formation of NH⋯OC hydrogen bonds and weaker interactions play a key role in the intermolecular recognition and assembly. Inter-annular CH⋯OC hydrogen bonds provide face to face or side by side arrangement of macrocycles in a way that can be considered the peptoid counterpart of β-sheet secondary structure in proteins. The choice of side chains is crucial for the solid state properties of α-cyclic peptoids. Side chains have a strong influence on the solid state assembly of peptoid macrocycles: they may provide competing interactions to CH⋯OC inter-annular hydrogen bonds, leading either to a T-shape or to a tubular arrangement of the peptoid macrocycles. The size of the macrocycle is another important factor influencing the tubular arrangement. In particular, a larger size of the macrocycle promotes side by side with respect to T-shape interactions. Hirshfeld surfaces and their fingerprint analysis allowed the analysis of the contributions of weak intermolecular interactions, such as weak CH⋯OC hydrogen bonds and CH–pi interactions, towards the crystal packing.
- This article is part of the themed collection: Structural Macrocyclic Supramolecular Chemistry

 Please wait while we load your content...
Please wait while we load your content...