Facile synthesis of Pd–Pt alloy concave nanocubes with high-index facets as electrocatalysts for methanol oxidation†
Abstract
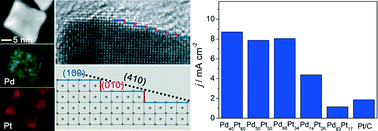
Pd–Pt alloy concave nanocubes enclosed by high-index facets were synthesized in ethylene glycol (EG) containing H2PtCl6 and Na2PdCl4 with ascorbic acid (AA) and KBr as reducing and capping agents, respectively. We found that the combination of bromide-induced galvanic replacement and co-reduction was responsible for the formation of these alloy concave nanocubes. The composition of the Pd–Pt alloy concave nanocubes was also tuned by varying the molar ratio of Pd to Pt salt precursors fed in the reaction. In addition, the variation in composition had a great impact on the rate of galvanic replacement, and thus the concave content of such alloy nanocrystals. These Pd–Pt alloy concave nanocubes showed composition-dependent catalytic activity for methanol oxidation, with Pd40Pt60-based catalysts exhibiting the highest activity. Compared to the commercial Pt/C, the Pd40Pt60 alloy concave nanocubes showed much improved tolerance toward CO poisoning, together with 4.6 times enhancement in specific activity for methanol oxidation due to possible synergetic effects between Pd and Pt and the unique surface structure associated with high-index facets.

 Please wait while we load your content...
Please wait while we load your content...