High solubility crystalline hydrates of Na and K furosemide salts†
Abstract
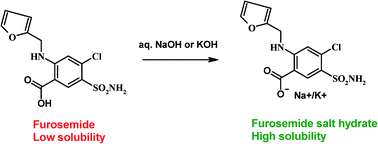
Novel sodium and potassium salts of the poorly soluble loop diuretic drug furosemide were prepared with the intent of improving drug solubility and bioavailability. Furo–Na salt was obtained as a trihydrate upon crystallization from aqueous NaOH solution, and furo–K salt crystallized as a monohydrate from KOH solution. Both salt hydrates were characterized by X-ray diffraction, DSC, TGA, and IR spectroscopy. An exothermic phase transition at 165 °C in the DSC heating curve of the furo–Na salt indicates the likelihood of polymorphism in its anhydrate phase. Based on solubility studies, furo–Na–trihydrate and furo–K–monohydrate in pH 7 phosphate buffer medium exhibited significantly higher aqueous solubilities of 41 mg mL−1 and 106 mg mL−1 compared to the free drug (0.01 mg mL−1). The physical stability of these fast dissolving salts under accelerated ICH conditions of 40 °C and 75% RH was modest, with furo–Na salt being stable for 2 weeks and furo–K salt for 1 week.
- This article is part of the themed collection: International Year of Crystallography Celebration: India

 Please wait while we load your content...
Please wait while we load your content...