Synthesis, characterization and selective hysteretic sorption property of metal–organic frameworks with 3,5-di(pyridine-4-yl)benzoate†
Abstract
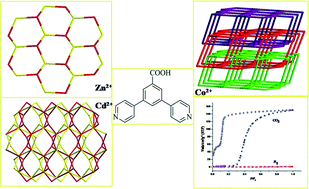
Three new metal–organic frameworks (MOFs) {[Zn(L)(HCOO)(H2O)]·H2O}n (1), {Cd(L)2}n (2) and {Co(L)(BDC)0.5(H2O)}n (3) [HL = 3,5-di(pyridine-4-yl)benzoic acid, H2BDC = 1,4-benzenedicarboxylic acid] were synthesized by hydro/solvothermal reactions and characterized by elemental analysis, thermogravimetric analysis, IR spectroscopy, powder and single crystal X-ray diffraction. Complex 1 is a two-dimensional (2D) network with {63} topology, which is further assembled into a three-dimensional (3D) supramolecular architecture through hydrogen bonding interactions. Complex 2 is a 2-fold interpenetrating 3D framework with a point (Schläfli) symbol of {63}{67·83}, while 3 is a 3D net formed by interpenetration of 2D bilayers (2D + 2D → 3D) with a point (Schläfli) symbol of {63}{66}. Selective and hysteretic sorption of carbon dioxide (CO2) was found for 3 at 195 K, and the photoluminescence properties of 1 and 2 were investigated.
- This article is part of the themed collection: International Year of Crystallography Celebration: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...