Highlights from the 49th EUCHEM Conference on Stereochemistry, Bürgenstock, Switzerland, May 2014
Gonçalo J. L.
Bernardes
*ab and
Andrew L.
Lawrence
*c
aDepartment of Chemistry, University of Cambridge, Lensfield Road, CB2 1EW Cambridge, UK. E-mail: gb453@cam.ac.uk
bInstituto de Medicina Molecular, Faculdade de Medicina da Universidade de Lisboa, Av. Prof. Egas Moniz, 1649-028 Lisboa, Portugal. E-mail: gbernardes@medicina.ulisboa.pt
cSchool of Chemistry, University of Edinburgh, Joseph Black Building, West Mains Road, Edinburgh, EH9 3JJ, UK. E-mail: a.lawrence@ed.ac.uk
First published on 11th August 2014
Arriving at the picturesque Seehotel Waldstätterhof in Brunnen on the shores of Lake Lucerne provides one with a distinct feeling of participating in a very special conference. Adding to this feeling of anticipation is the question everyone is asking in the hotel foyer; do you know who is speaking? The ‘Bürgenstock Conference’, or more officially the ‘EUCHEM Conference on Stereochemistry’, is a conference steeped in tradition with many time-honoured rules. One of these rules is that the line up of 14 speakers is not published ahead of the meeting, thus creating a real buzz among the participants. The president of the 2014 Bürgenstock Conference, Antonio Echavarren (Institute of Chemical Research of Catalonia) and the organizing committee Alain De Mesmaeker (Syngenta Crop Protection Research), Jérôme Lacour (University of Geneva), Reto Naef (Novartis Pharma AG), Philippe Renaud (University of Bern) and Helma Wennemers (ETH Zürich) did not disappoint in their selection of speakers from across the world. The breadth of subjects that was to be covered is a hallmark of the Bürgenstock Conference, thus offering everybody in attendance an opportunity to learn about areas outside their personal expertise. At dinner, the president welcomed everybody to the Conference from the stone balcony overlooking the dining hall, which has panoramic views over Lake Lucerne (Fig. 1). It was here that Antonio announced that the Guest of Honour for the 49th Bürgenstock Conference was Javier De Mendoza (Institute of Chemical Research of Catalonia), who had himself been president of the Conference in 1999. Antonio then explained the personal impact Javier had made on his life and academic career. | ||
| Fig. 1 The view over Lake Lucerne from the lakeside terrace at the Seehotel Waldstätterhof. Image kindly provided by Carla Obradors (Institute of Chemical Research of Catalonia). | ||
The science commenced after dinner with a brilliant talk entitled Tunneling Control of Chemical Reactions by Peter Schreiner (Justus Liebig-Universität Gießen). Peter opened his presentation by discussing classical transition state theory and the associated kinetic and thermodynamic control of reactions, which we are all familiar with. Peter then provided exemplar situations that are under neither kinetic nor thermodynamic control but are instead under what he terms ‘tunneling control’.1 Noble gas matrices are the perfect environment in which to study these reactions and examples were provided wherein key intermediates were generated using flash vacuum pyrolysis and then trapped in low temperature matrices. A take home message was that the rate of tunneling is directly proportional to the width of the barrier whilst only proportional to the square root of the height, i.e., tall energy barriers that are narrow can result in processes under ‘tunneling control’.2 Questions of inducing enantioselectivity were raised after the lecture and the ensuing discussion included reference to enzyme catalysis, perhaps pointing towards a change in the widely accepted dogma that only kinetic control, understood through classical transition state theory, can lead to selectivity. Needless to say, this fascinating talk was discussed long into the evening in the charming hotel bar.
Monday morning began with a fascinating talk from Christophe Copéret (ETH Zürich) concerning his group's efforts to bring a molecular level of understanding and design to heterogeneous catalysis.3 Christophe started by presenting the established heterogeneous and homogeneous alkene metathesis catalysts and described their associated advantages and disadvantages. The Copéret Group work on the development of Surface Organometallic Chemistry (SOMC) to enable the production of heterogeneous catalysts with a specific density of well-defined single-site functionalities. With the knowledge of the type and number of surface silanol groups present, silica can be used to create such well-defined heterogeneous catalysts. Through this approach the Copéret Group were able to synthesize and characterize a highly active and well-defined rhenium heterogeneous alkene metathesis catalyst.4 With this lead-catalyst in hand the development of higher generation catalysts was possible through computational investigations.5 The development and understanding of other heterogeneous catalysts was then presented, including the Phillips chromium ethylene polymerization catalyst. The second half of the lecture was devoted to advances made in Surface Enhanced NMR Spectroscopy by Dynamic Nuclear Polarization (DNP-SENS).6,7 Listening to the talk it became very clear that the success of this research was only possible through a highly effective dialogue between researchers working on chemical reactivity, spectroscopy and computational studies.
The second lecture of the morning was given by Anna Mapp (University of Michigan) who highlighted the discovery of small molecule transcriptional modulators using a Tethering strategy. This is a powerful approach to the discovery of ligands that disrupt protein–protein interactions (PPIs), which are strongly correlated with human disease. The Tethering strategy consists of screening a library of disulfide-containing fragments under reversible conditions against a protein target that is equipped with an engineered cysteine adjacent to the binding site. These efforts have led, for instance, to the discovery of small-molecule ligands for the GACKIX domain of the master coactivator CBP/p300. In addition, it was possible to identify a small molecule that when covalently linked to the GACKIX domain has enabled a high-resolution snapshot of the coactivator interacting with a ligand.8 Thus, Tethering may facilitate the structural characterization of conformationally dynamic coactivator domains complexed with small molecule ligands to guide the design of more potent inhibitors of PPIs using rational structure-based approaches. Furthermore, Anna Mapp presented the identification of two natural product ligands, sekikaic acid and lobaric acid, that specifically target a dynamic surface of the CBP/p300 GACKIX domain. This research highlights the potential of natural products for targeting specific PPIs.9 Following the discussion session, everyone assembled on the sunny lakeside terrace for the official conference photo before having lunch in the main dinning hall.
The afternoons at Bürgenstock Conferences are generally free for the attendees to enjoy the town of Brunnen and to tackle some of the local hiking trails. Monday and Thursday afternoons are, however, cut a little short with the very well attended pre-dinner poster sessions. A total of 42 posters were presented, split evenly over the two days, and the organizing committee selected five presenters in each session to provide short appetizer talks for their posters. On Monday, John Bower (University of Bristol), Dennis Gillingham (University of Basel), Nathalie Katsonis (University of Twente), Andrew Lawrence (University of Edinburgh) and Daniel Summerer (University of Konstanz) presented their short talks in the lecture hall before participating in an excellent poster session.
Following dinner, everyone assembled in the lecture hall for the evening lecture, which was to be presented by Kenichiro Itami (Nagoya University). Before this lecture, however, Wolf-Dietrich Woggon (Universität Basel) presented a tribute to the founder and first president of the Bürgenstock Conference, André Dreiding, who had sadly died on 24th December 2013.10 It was a fascinating and insightful personal tribute to a great scientist, without whom we would not have this fantastic conference. Kenichiro then took to the stage to impress the audience with his exciting contributions to both biology and materials science, enabled by ingenious application of catalysis and C–H activation chemistry. One of the research themes presented was the bottom-up approach towards nano-carbon structures. A particularly striking example was the synthesis of grossly warped nanographene (Fig. 2), which was accessed during attempts to annulate corannulene using C–H activation chemistry.11 As well as being a powerful demonstration of this emerging area of synthetic chemistry, this work also allowed investigation of the physical changes observed when odd membered rings are introduced into nanographene structures. Kenichiro was quick to point out that it was others who had dubbed his product the “Graphene Pringle”!
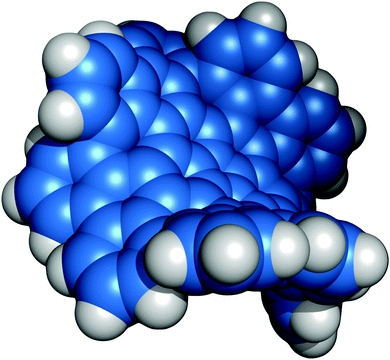 | ||
| Fig. 2 ORTEP image showing the warped structure of Itami's C80H30 nanographene, taken from the X-ray crystal structure. Figure kindly provided by Kenichiro Itami (Nagoya University). | ||
Tuesday morning began with another talk focused on C–H activation chemistry by Naoto Chatani (Osaka University), which described his group's research on directed functionalisations using bidentate directing groups.12 The research described focused on the fundamental development of catalytic C–H bond activation chemistry with new reactivity and mechanistic insights clearly of upmost importance for the Chatani Group. The power of bidentate directing groups was illustrated with the Ru catalyzed C–H carbonylation reaction. This reaction was first achieved in 1997 using a monodentate pyridine directing group to activate ortho-aromatic C(sp2)–H bonds.13 With the aid of bidentate directing groups the Chatani Group have now extended this chemistry to activate the far more challenging C(sp3)–H bonds.14 Mechanistic details of both Ru(II) and Ni(II) catalyzed C–H bond activation reactions were then discussed and exciting new methylation, alkylation and arylation reactions of C–H bonds were presented.
Marcey Waters (University of North Carolina at Chapel Hill) delivered the second morning lecture of Tuesday on chemical tools to understand recognition of histone protein modifications in nature. The Waters Group explores the use of dynamic combinatorial chemistry (DCC) to develop small molecule receptors that are able to sense post-translational modifications (PTMs). These efforts led to the identification of a synthetic receptor for trimethyl lysine (KMe3) that mimics, with comparable affinity and selectivity, the native HP1 chromodomains.15 Minimal structural alterations of the scaffold allowed development of a new synthetic receptor that binds asymmetric dimethyl arginine (aRMe2). The affinity and selectivity for arginine methylation was found to be identical to that of proteins that bind to these PTMs.16 In both cases, a cation–π interaction was found to contribute significantly to the observed binding of the synthetic receptor and the PTM.
In the evening John Porco (Boston University) presented a fascinating lecture on natural product synthesis. John described his group's unified biomimetic approach to the aglain, forbaglin, and rocaglamide classes of natural products which utilizes a highly unusual excited-state intramolecular proton transfer (ESIPT) reaction. 3-Hydroxyflavones undergo ESIPT to generate oxidopyrylium ylides that then undergo [3+2] cycloadditions with cinnamate derivatives to afford the aglain core. The Porco Group then used basic conditions to promote the required α-ketol rearrangement to afford the rocaglamide core or oxidative conditions to afford the forbaglin framework.17 Use of chiral Brønsted acids (TADDOLs) then allowed for an enantioselective [3+2] cycloaddition thus providing an enantioselective approach towards the rocaglamides.18 Biological studies were then enabled by having ready access to rocaglates which are inhibitors of translation initiation and heat shock factor 1 (HSF1) activation.19
The morning of the fourth day of the conference led us into the world of chemical biology and began with a spectacular lecture by Chris Chang (University of California, Berkeley) on the design and synthesis of molecular imaging probes and their use to discover and understand the chemistry of the brain. Chris's lab has constructed a number of synthetic probes that allow selective tracking of biologically relevant molecules including H2O2, CO, H2S and reactive oxygen species (ROS) in live cells.20 Upon chemoselective reaction, the probes turn fluorescent allowing visualisation of these molecules in their native environment. In this way, it is possible to unravel the roles of these signalling molecules in physiological and pathological relevant systems. For instance, a sulfidefluor-7-acetoxymethyl ester probe has enabled chemoselective, real-time visualisation of endogenous H2S produced in live human umbilical vein endothelial cells upon stimulation with vascular endothelial growth factor (VEGF).21 Furthermore, H2S production was shown to be dependent on a H2O2 signal strongly suggesting H2S/H2O2 crosstalk. In another example, using peroxyfluor-6 (PF6), a selective fluorescent indicator for hydrogen peroxide (H2O2), it was shown that NADPH oxidase 2 (Nox2) redox signalling through controlled ROS chemistry is essential to preserve key cell populations in the brain.22 These examples illustrate the utility of chemoselective probes for selective molecular imaging of biologically relevant molecules in their native environments.
The second lecture of Wednesday morning was given by John Sutherland (MRC Laboratory of Molecular Biology, Cambridge) who gave a fascinating overview of prebiotically plausible chemistry of the molecules necessary for the origin of life. The synthesis of pyrimidine ribonucleoside-2′,3′-cyclic phosphates has been demonstrated using starting materials (e.g., cyanamide, cyanoacetylene, glycoaldehyde, glyceraldehyde and inorganic phosphate) in conditions consistent with geochemical models in the early-Earth.23 This discovery suggested the involvement of ribonucleic acid (RNA) at an initial stage in the origins of life. The Sutherland Group have now shown that the 2′-hydroxyl group of oligoribonucleotide-3′-phosphates can be chemoselectively acetylated in aqueous and prebiotically plausible conditions (Fig. 3).24 The 2′-O-acetyl group needed for the template-directed ligation can then be removed using mild conditions that maintain the new 3′,5′-internucleotide linkages intact. This work suggests the participation of 2′-O-acetylated RNA in the prebiotic conversion of ribonucleoside-2′,3′-cyclic phosphates to 3′,5′-linked RNA.
 | ||
| Fig. 3 Site-selective acylation of the 2′-hydroxyl group promotes rapid template-directed 3′,5′-ligation after electrophilic phosphate activation. Figure adapted from ref. 24. | ||
Instead of a scientific lecture, Wednesday evening was devoted to music, this year presented by the Asasello Quartet, followed by an evening get-together with the musicians: Rostislav Kozhevnikov (violin), Barbara Kuster (violin), Justyna Śliwa (viola) and Teemu Myöhänen (cello).
Thursday morning began with a highly thought-provoking lecture by Armido Studer (Westfälische Wilhelms-Universität Münister). The lecture was an overview of several areas of beautiful free radical chemistry. Armido had an overarching aim to convince the audience that the electron is a catalyst, with analogies drawn to how the proton is often considered a catalyst. Specific areas of chemistry discussed included base-promoted homolytic aromatic substitution reactions (BHAS),25 novel radical trifluoromethylation reactions26 and the use of TEMPO in redox chemistry.27,28 The presentation of proposed mechanisms for homolytic aromatic substitution reactions was very interesting and in the question session John Murphy (University of Strathclyde) proposed a bet of 50 Swiss francs that his recently published29 proposed initiation mechanism for BHAS reactions would be shown to be correct. The rest of the discussion session was equally interesting with questions raised regarding the idea of the electron as a catalyst and the use of the term transition metal free.
This was followed by an outstanding lecture from Michelle Chang (University of California, Berkeley) on synthetic biology approaches to new chemistry. A detailed understanding of the molecular mechanisms living cells use to control enzymatic processes within the context of the entire metabolic network has enabled the Chang Group to engineer new biosynthetic pathways in microbial hosts for in vivo production of fluorinated natural products30 and biofuels.31 In particular, engineered polyketide synthase pathways in Escherichia coli bacteria were used as a source of fluorinated building blocks to incorporate fluorine into polyketide products site-selectively in vitro and in vivo (Fig. 4A).30 In addition, by reconstructing the synthetic pathway for 1-butanol (an attractive biofuel with high energy content) the Chang Group were able to assemble a very efficient chimeric synthetic pathway in Escherichia coli for the production of 1-butanol (Fig. 4B).31
 | ||
| Fig. 4 (A) Production of fluorinated polyketides in vitro: reaction catalysed by the sixth module of 6-deoxyerythronolide B synthase (DEBSMod6) + trans-enoyl-CoA reductase (Ter) using a diketide substrate [natural diketide N-acetyl cysteamine thioesterase (NDK-SNAC)]. Figure adapted from ref. 30. (B) An efficient chimeric synthetic pathway in Escherichia coli for the production of 1-butanol. Figure adapted from ref. 31. CoA = coenzyme A, NADH = nicotinamide adenine dinucleotide (reduced form), TE = terminal thioesterase. | ||
Thursday afternoon saw the second of the two poster sessions, with Jonathan George (University of Adelaide), Bernhard Kräutler (University of Innsbruck), Clement Mazet (University of Geneva), Raphaël Rodriguez (CNRS, ICSN in Gif-sur-Yvette), Daniel Strand (Lund University) and Oliver Trapp (Universität Heidelberg) selected to present short appetizer talks in the lecture hall before participating in another excellent pre-dinner poster session.
The Thursday evening lecture was presented by Nazario Martín (University Complutense of Madrid) and detailed the use of concave–convex supramolecular interactions in carbon nanoforms. Aggregation is a common problem when dealing with carbon nanoforms and much research has been directed to the use of covalent chemistry to address this issue. The Martín Group were inspired by the occurrence of carbon nano-onions to develop a supramolecular solution to this problem. The Martín Group have developed the use of π-extended analogues of tetrathiafulvalene, namely 2-[9-(1,3-dithiol-2-ylidene)]-1,3-dithiole (exTFF) units, as the basis for supramolecular receptors for fullerenes and other nanoforms of carbon.32 The exTFF receptor is based on π–π and van der Waals interactions between the concave face of the exTFF moieties and the convex exterior of fullerenes. This unique receptor has allowed the development of molecular tweezers for C60,33 the construction of redox-amphoteric supramolecular polymers34 and most recently the synthesis of novel mechanically interlocked carbon nanotubes (MINTs).35
The final day of the conference commenced with Jesus Jiménez-Barbero giving a master-class presentation on the use of NMR to study the vitally important interactions between proteins and carbohydrates, which are key to a variety of molecular recognition events. The audience was first introduced to the significant issues associated with studying these interactions using NMR spectroscopy, as the carbohydrate ligands behave very differently to the large receptors and receptor–ligand complexes.36 Two NMR techniques were described, Saturation-Transfer-Difference NMR (STD-NMR) and Transferred NOESY NMR (TR-NOESY), which interrogate these important interactions from the ligand's perspective. Both techniques rely on the nuclear Overhauser effect to determine the binding epitope of ligands (the hydrogens of the ligand that are closest to the protein upon binding).3715N–1H HSQC NMR experiments were then described that are used to analyze protein–ligand interactions from the perspective of the receptor. Using these sophisticated NMR experiments the Jiménez-Barbero Group were not only able to analyze ligands with single epitopes but more complex molecules with multiple epitopes, thus allowing insight into lectin selectivity.38 Jesus’ presentation also included a fascinating introduction into the development of new methodologies, based on paramagnetic lanthanides, to unravel conformational aspects and detect elusive protein–carbohydrate interactions.39,40
The final lecture of the 2014 Bürgenstock conference was delivered by Matthew Sigman (University of Utah). The lecture focused on analyzing selectivity in asymmetric catalysis with the ultimate aim of providing more accurate and reliable predictions.41 The Sigman Group follows a very specific approach, wherein multiple reaction variables are analyzed simultaneously. For instance, development of asymmetric propargylation was enabled through three-dimensional correlation of ligand steric and electronic free energy relationships.42 Matthew described how the simultaneous analysis of multiple variables in catalytic processes is an enabling approach to the development of new asymmetric catalytic methods. The very nature of synthetic chemistry means that any new synthetic method that is going to be widely adopted by the synthetic community needs to have high selectivity and, crucially, broad substrate scope. By applying their multifaceted approach they were able to simultaneously analyze both catalyst and substrate steric effects on the enantioselective propargylation reaction.43 Although challenging, it is clear that this approach has the power to offer distinct predictive advantages to the synthetic chemist and can offer greater insight into important and often subtle ligand features for asymmetric induction. This concluded the scientific content of the 49th Bürgenstock Conference. The president, Antonio Echavarren, formally brought proceedings to a close, passing on the responsibility for organizing the 2015 Bürgenstock conference to Antonio Togni (ETH Zürich). Everyone then had a chance to say their goodbyes over lunch before heading their separate ways.
G.J.L.B. is a Royal Society University Research Fellow at the Department of Chemistry, University of Cambridge and a Principal Investigador FCT at the Instituto de Medicina Molecular, Lisbon.
A.L.L. is a lecturer at the School of Chemistry, University of Edinburgh and adjunct research fellow at the Research School of Chemistry, Australian National University. A.L.L. thanks the Swiss Chemical Society (SCS), the Fonds der Chemischen Industrie (FCI) and the Bürgenstock organising committee for the award of a JSP Fellowship.
Notes and references
- For an example, see: P. R. Schreiner, H. P. Reisenauer, D. Ley, D. Gerbig, C.-H. Wu and W. D. Allen, Science, 2011, 332, 1300–1303 CrossRef CAS PubMed.
- D. Ley, D. Gerbig and P. R. Schreiner, Org. Biomol. Chem., 2012, 10, 3781–3790 CAS.
- M. P. Conley, M. F. Delley, G. Siddiqi, G. Lapadula, S. Norsic, V. Monteil, O. V. Safonova and C. Copéret, Angew. Chem., Int. Ed., 2014, 53, 1872–1876 CrossRef PubMed.
- M. Chabanas, A. Baudouin, C. Copéret and J.-M. Basset, J. Am. Chem. Soc., 2001, 123, 2062–2063 CrossRef CAS.
- For example, see: F. Blanc, J. Thivolle-Cazat, J.-M. Basset, C. Copéret, A. S. Hock, Z. J. Tonzetich, A. Sinha and R. R. Schrock, J. Am. Chem. Soc., 2007, 129, 1044–1045 CrossRef CAS PubMed.
- A. Rossini, A. Zagdoun, M. Lelli, A. Lesage, C. Copéret and L. Emsley, Acc. Chem. Res., 2013, 46, 1942–1951 CrossRef CAS PubMed.
- D. Gajan, M. Schwarzwälder, M. P. Conley, W. R. Gruening, A. J. Rossini, A. Zagdoun, M. Lelli, M. Yulikov, G. Jeschke, C. Sauvée, O. Ouari, P. Tordo, L. Veyre, A. Lesage, C. Thieuleux, L. Emsley and C. Coperet, J. Am. Chem. Soc., 2013, 135, 15459–15466 CrossRef CAS PubMed.
- N. Wang, C. Y. Majmudar, W. C. Pomerantz, J. K. Gagnon, J. D. Sadowsky, J. L. Meagher, T. K. Johnson, J. A. Stuckey, C. L. Brooks III, J. A. Wells and A. K. Mapp, J. Am. Chem. Soc., 2013, 135, 3363–3366 CrossRef CAS PubMed.
- C. Y. Majmudar, J. W. Højfeldt, C. J. Arevang, W. C. Pomerantz, J. K. Gagnon, P. J. Schultz, L. C. Cesa, C. H. Doss, S. P. Rowe, V. Vásquez, G. Tamayo-Castillo, T. Cierpicki, C. L. Brooks, D. H. Sherman and A. K. Mapp, Angew. Chem., Int. Ed., 2012, 51, 11258–11262 CrossRef CAS PubMed.
- W.-D. Woggon, Chimia, 2014 DOI:10.2533/chimia.2014.575.
- K. Kawasumi, Q. Zhang, Y. Segawa, L. T. Scott and K. Itami, Nat. Chem., 2013, 5, 739–744 CrossRef CAS PubMed.
- G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726–11743 CrossRef CAS PubMed.
- N. Chatani, Y. Ie, F. Kakiuchi and S. Murai, J. Org. Chem., 1997, 62, 2604–2610 CrossRef CAS PubMed.
- N. Hasegawa, V. Charra, S. Inoue, Y. Fukumoto and N. Chatani, J. Am. Chem. Soc., 2011, 133, 8070–8073 CrossRef CAS PubMed.
- L. A. Ingerman, M. E. Cuellar and M. L. Waters, Chem. Commun., 2010, 46, 1839–1841 RSC.
- L. I. James, J. E. Beaver, N. W. Rice and M. L. Waters, J. Am. Chem. Soc., 2013, 135, 6450–6455 CrossRef CAS PubMed.
- B. Gerard, G. Jones II and J. A. Porco Jr., J. Am. Chem. Soc., 2004, 126, 13620–13621 CrossRef CAS PubMed.
- B. Gerard, S. Sangji, D. O'Leary and J. A. Porco Jr., J. Am. Chem. Soc., 2006, 128, 7754–7755 CrossRef CAS PubMed.
- S. Santagatal, M. L. Mendillo, Y.-c. Tang, A. Subramanian, C. C. Perley, S. P. Roche, B. Wong, R. Narayan, H. Kwon, M. Koeva, A. Amon, T. R. Golub, J. A. Porco Jr, L. Whitesell and S. Lindquist, Science, 2013, 341, 1238303 CrossRef PubMed.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed.
- V. S. Lin, A. R. Lippert and C. J. Chang, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 7131–7135 CrossRef CAS PubMed.
- B. C. Dickinson, J. Peltier, D. Stone, D. V. Schaffer and C. J. Chang, Nat. Chem. Biol., 2011, 7, 106–112 CrossRef CAS PubMed.
- M. W. Powner, B. Gerland and J. D. Sutherland, Nature, 2009, 459, 239–242 CrossRef CAS PubMed.
- F. R. Bowler, C. K. W. Chan, C. D. Duffy, B. Gerland, S. Islam, M. W. Powner, J. D. Sutherland and J. Xu, Nat. Chem., 2013, 5, 383–389 CrossRef CAS PubMed.
- A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2011, 50, 5018–5022 CrossRef CAS PubMed.
- B. Zhang, C. Mück-Lichtenfeld, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2013, 52, 10792–10795 CrossRef CAS PubMed.
- Y. Li and A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8221–8224 CrossRef CAS PubMed.
- M. Hartmann, Y. Li and A. Studer, J. Am. Chem. Soc., 2012, 134, 16516–16519 CrossRef CAS PubMed.
- S. Zhou, G. M. Anderson, B. Mondal, E. Doni, V. Ironmonger, M. Kranz, T. Tuttle and J. A. Murphy, Chem. Sci., 2014, 5, 476–482 RSC.
- M. C. Walker, B. W. Thuronyi, L. K. Charkoudian, B. Lowry, C. Khosla and M. C. Y. Chang, Science, 2013, 341, 1089–1094 CrossRef CAS PubMed.
- B. B. Bond-Watts, R. J. Bellerose and M. C. Y. Chang, Nat. Chem. Biol., 2011, 7, 222–227 CrossRef CAS PubMed.
- E. M. Pérez and N. Martín, Chem. Soc. Rev., 2008, 37, 1512–1519 RSC.
- E. M. Pérez, L. Sánchez, G. Fernández and N. Martín, J. Am. Chem. Soc., 2006, 128, 7172–7173 CrossRef PubMed.
- G. Fernández, E. M. Pérez, L. Sánchez and N. Martín, Angew. Chem., Int. Ed., 2008, 47, 1094–1097 CrossRef PubMed.
- A. de Juan, Y. Pouillon, L. Ruiz-González, A. Torres-Pardo, S. Casado, N. Martín, Á. Rubio and E. M. Pérez, Angew. Chem., Int. Ed., 2014, 53, 5394–5400 CrossRef CAS PubMed.
- J. Jiménez-Barbero, F. J. Cañada, J. L. Asensio, N. Aboitiz, P. Vidal, A. Canales, P. Groves, H.-J. Gabius and H. C. Siebert, Adv. Carbohydr. Chem. Biochem., 2006, 60, 303–354 CrossRef.
- J. J. Hernández-Gay, A. Ardá, S. Eller, S. Mezzato, B. R. Leeflang, C. Unverzagt, F. J. Cañada and J. Jiménez-Barbero, Chem. - Eur. J., 2010, 16, 10715–10726 CrossRef PubMed.
- A. Ardá, P. Blasco, S. D. Varón, V. Schubert, S. André, M. Bruix, F. J. Cañada, H. J. Gabius, C. Unverzagt and J. Jiménez-Barbero, J. Am. Chem. Soc., 2013, 135, 2667–2675 CrossRef PubMed.
- A. Canales, A. Mallagaray, J. Pérez-Castells, I. Boos, C. Unverzagt, S. André, H.-J. Gabius, F. J. Cañada and J. Jiménez-Barbero, Angew. Chem., Int. Ed., 2013, 52, 13789–13793 CrossRef CAS PubMed.
- A. Canales, A. Mallagaray, M. A. Berbís, A. Navarro-Vázquez, G. Domí, F. J. Cañada, S. André, H.-J. Gabius, J. Pérez-Castells and J. Jiménez-Barbero, J. Am. Chem. Soc., 2014, 136, 8011–8017 CrossRef CAS PubMed.
- K. C. Harper and M. S. Sigman, J. Org. Chem., 2013, 78, 2813–2818 CrossRef CAS PubMed.
- K. C. Harper and M. S. Sigman, Science, 2011, 333, 1875–1878 CrossRef CAS PubMed.
- K. C. Harper, S. C. Vilardi and M. S. Sigman, J. Am. Chem. Soc., 2013, 135, 2482–2485 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |
