Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Regio- and stereoselective synthesis of benzothiazolo-pyrimidinones via an NHC-catalyzed Mannich/lactamization domino reaction†
Qijian
Ni
,
Xiaoxiao
Song
,
Jiawen
Xiong
,
Gerhard
Raabe
and
Dieter
Enders
*
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany. E-mail: enders@rwth-aachen.de
First published on 26th November 2014
Abstract
An NHC-catalyzed regio- and stereoselective Mannich/lactamization domino reaction of N-(benzothiazolyl)imines with α-chloroaldehydes has been developed. This new protocol provides a facile approach for the asymmetric synthesis of benzothiazolo-pyrimidinones and a pyrrolo[1,2-a]indolone in moderate to good yields (34–78%) and excellent stereoselectivities (87–99% ee, up to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r.).
1 d.r.).
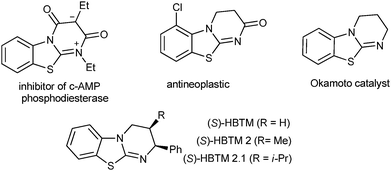
The tricyclic pyrimido[2,1-b]benzothiazole core prevails in a wide range of bioactive molecules with remarkable biological properties,1 such as the inhibition of c-AMP phosphodiesterase, antineoplastic and antimalarial activity. Moreover, the isothiourea-based HBTM is used as an efficient organocatalyst and received great attention in the field of asymmetric catalysis (Fig. 1).2 Although various approaches for the synthesis of the pyrimido[2,1-b]benzothiazole motif have been developed, most of them are non-stereoselective and/or need relatively harsh conditions.3
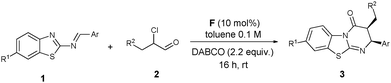
In the past decade, great advances have been achieved in the development of N-heterocyclic carbene (NHC) catalyzed organocatalytic reactions via the umpolung of aldehydes.4 Especially since the seminal studies concerning the NHC-catalyzed conjugate umpolung reactions reported by the groups of Glorius and Bode in 2004,5 NHC organocatalysis has been extended for the activation of the β-carbon (homoenolate intermediate) and α-carbon (enolate intermediate) of enals. These two kinds of intermediates used as nucleophiles reacted with a variety of reactive electrophiles to afford heterocyclic compounds such as lactones, lactams or cyclopentenes.6 It is noteworthy that aldimines behaved as excellent electrophiles in the reactions of enolate intermediates, providing the corresponding β-lactams (Scheme 1, eqn (1)). Smith et al.7 and Ye et al.8 reported an NHC-catalyzed [2+2] cycloaddition of ketenes with N-tosyl imines and N-Boc imines, respectively. Very recently, the Ye group was able to carry out Staudinger reactions of ketenes with isatin-derived ketimines, yielding spirocyclic oxindolo-β-lactams.9 We envisioned that 2-benzothiazolimine 1a in combination with an azolium enolate could not only produce the β-lactam 4, but also provide access to benzothiazolo-pyrimidinone 3 through a formal [4+2] annulation (Scheme 1, eqn (2)). Obviously, influencing the regioselectivity of the reaction site of the intermediate ambident anions is the greatest challenge in order to improve the ratio of 3/4 in the Mannich/lactamization domino reaction.
To test our hypothesis, we initially checked several triazolium precatalysts A–C in the model reaction of 2-benzothiazolimine 1a with 2-chloro-3-phenylpropanal (2a) at room temperature. We found that the chiral triazolium salt B resulted in an excellent stereoselectivity for ent-3a (97% ee, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r.), albeit with a low yield (19%) and regioselectivity (Table 1, entry 2). Attempting to improve the regioselectivity and the yield of ent-3a, we next tested a series of bases, but no satisfying improvement was achieved (Table 1, entries 4–9). Solvent screening led to no improvement in yield and selectivity (Table 1, entries 10–14). After the screening of the base and solvent, DABCO in combination with toluene turned out to give the highest yield of ent-3a (31%), maintaining the excellent stereoselectivity and the low regioselectivity as well (Table 1, entry 6). Notably, the triazolium salt A provided rac-3a in 43% yield exclusively, even though with a drastically decreased d.r. (Table 1, entry 1). Therefore we further screened a series of pyrrolidinone-derived triazolium salts D–G. To our delight, a dramatic improvement in both yield and regioselectivity was obtained (Table 1, entries 15–18). The NHC-catalyzed reaction based on the triazolium salt F afforded cycloadduct 3a in a better yield but with relatively low regioselectivity (Table 1, entry 17). After using 4 Å MS as an additive, the yield (69%) and regioselectivity (14
1 d.r.), albeit with a low yield (19%) and regioselectivity (Table 1, entry 2). Attempting to improve the regioselectivity and the yield of ent-3a, we next tested a series of bases, but no satisfying improvement was achieved (Table 1, entries 4–9). Solvent screening led to no improvement in yield and selectivity (Table 1, entries 10–14). After the screening of the base and solvent, DABCO in combination with toluene turned out to give the highest yield of ent-3a (31%), maintaining the excellent stereoselectivity and the low regioselectivity as well (Table 1, entry 6). Notably, the triazolium salt A provided rac-3a in 43% yield exclusively, even though with a drastically decreased d.r. (Table 1, entry 1). Therefore we further screened a series of pyrrolidinone-derived triazolium salts D–G. To our delight, a dramatic improvement in both yield and regioselectivity was obtained (Table 1, entries 15–18). The NHC-catalyzed reaction based on the triazolium salt F afforded cycloadduct 3a in a better yield but with relatively low regioselectivity (Table 1, entry 17). After using 4 Å MS as an additive, the yield (69%) and regioselectivity (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) improved further, but the enantioselectivity (93% ee) decreased slightly (Table 1, entry 19).
1) improved further, but the enantioselectivity (93% ee) decreased slightly (Table 1, entry 19).
| Entry | NHC | Base | Solvent | Yield of 3ab (%) | 3a/4ac | d.r. (3a)d | ee of 3ae (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.2 mmol), NHC (0.01 mmol), base (0.22 mmol), solvent (1 mL), rt, 16 h. b Yields of isolated 3a after flash column chromatography. c Ratio based on isolated yields. d Determined by 1H NMR. e The ee value was determined by HPLC on a chiral stationary phase. f Addition of 4 Å MS. DIPEA = N,N-diisopropylethylamine, TMEDA = tetramethylethylenediamine, DABCO = 1,4-diazabicyclo[2.2.2]octane, DBU = 1,8-diazabicyclo-[5.4.0]undec-7-ene, Mes = 2,4,6-trimethylphenyl, TBDPS = tert-butyldiphenylsilyl, and TIPS = triisopropylsilyl. | |||||||
| 1 | A | NEt3 | Toluene | 43 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— |
| 2 | B | NEt3 | Toluene | 19 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−97 |
| 3 | C | NEt3 | Toluene | — | — | — | — |
| 4 | B | DIPEA | Toluene | 30 | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−99 |
| 5 | B | TMEDA | Toluene | 23 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−98 |
| 6 | B | DABCO | Toluene | 31 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−94 |
| 7 | B | DBU | Toluene | — | — | — | — |
| 8 | B | K2CO3 | Toluene | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−97 |
| 9 | B | NaOAc | Toluene | — | — | — | — |
| 10 | B | DABCO | THF | 24 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−99 |
| 11 | B | DABCO | DCM | 22 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−98 |
| 12 | B | DABCO | EtOAc | 30 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−99 |
| 13 | B | DABCO | PhCl | 14 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−98 |
| 14 | B | DABCO | Dioxane | 22 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−97 |
| 15 | D | DABCO | Toluene | 27 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−90 |
| 16 | E | DABCO | Toluene | 58 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
| 17 | F | DABCO | Toluene | 65 | 7.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 |
| 18 | G | DABCO | Toluene | 54 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−92 |
| 19f | F | DABCO | Toluene | 69 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
Next we investigated the substrate scope of this protocol on a 0.5 mmol scale. As shown in Table 2, a wide range of 2-benzothiazolimines 1 with diverse electronic and steric properties were first explored. The use of 2-benzothiazolimines 1a afforded the desired 3a in 63% yield with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r. and 90% ee (Table 2, 3a). Electron-donating substituents such as 4-Me and 4-OMe on the Ar group reduced the electrophilicity of the imine carbon, which led to lower yields and even the diasteroselectivity in 3c (Table 2, 3b,c). In the case of electron-withdrawing groups such as 4-Br, 4-Cl and 2-Cl, the reactions gave the desired cycloadducts 3d–f in good yields and with good to excellent diastereo- and enantioselectivities. The introduction of a heterocyclic furyl group on the Ar position gave compatible results, affording the corresponding product 3g in 69% yield, 11
1 d.r. and 90% ee (Table 2, 3a). Electron-donating substituents such as 4-Me and 4-OMe on the Ar group reduced the electrophilicity of the imine carbon, which led to lower yields and even the diasteroselectivity in 3c (Table 2, 3b,c). In the case of electron-withdrawing groups such as 4-Br, 4-Cl and 2-Cl, the reactions gave the desired cycloadducts 3d–f in good yields and with good to excellent diastereo- and enantioselectivities. The introduction of a heterocyclic furyl group on the Ar position gave compatible results, affording the corresponding product 3g in 69% yield, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r. and 93% ee under an elevated temperature. Several electron-donating and electron-withdrawing substituents such as R1 were also investigated, yielding the cycloadducts 3h–k in good yields and excellent stereoselectivities. We then varied the α-chloroaldehyde moiety. An aliphatic linear α-chloroaldehyde reacted smoothly with a slight loss of yield and with a reasonable ee value (Table 2, 3l). When a para-nitrophenyl group instead of Ph as R2 was used, a better result in terms of yield and ee was obtained (Table 2, 3m).
1 d.r. and 93% ee under an elevated temperature. Several electron-donating and electron-withdrawing substituents such as R1 were also investigated, yielding the cycloadducts 3h–k in good yields and excellent stereoselectivities. We then varied the α-chloroaldehyde moiety. An aliphatic linear α-chloroaldehyde reacted smoothly with a slight loss of yield and with a reasonable ee value (Table 2, 3l). When a para-nitrophenyl group instead of Ph as R2 was used, a better result in terms of yield and ee was obtained (Table 2, 3m).
| 3 | R1 | Ar | R2 | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.5 mmol), 2 (1.0 mmol), F (0.05 mmol), DABCO (1.1 mmol), toluene (5 mL), 4 Å MS, rt, 16 h. b Yield of isolated 3 after flash column chromatography. c Determined by 1H NMR. d The ee value was determined by HPLC on a chiral stationary phase. e The reaction time is 24 h. f Performed at 40 °C. g The reaction time is 48 h. | ||||||
| a | H | Ph | Ph | 63 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
| b | H | 4-MePh | Ph | 49 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| c | H | 4-MeOPh | Ph | 34 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| d | H | 4-BrPh | Ph | 56 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| e | H | 4-ClPh | Ph | 61 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
89 |
| f | H | 2-ClPh | Ph | 60 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 |
| g | H | 2-Furyl | Ph | 69 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| h | Me | Ph | Ph | 64 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| i | MeO | Ph | Ph | 56 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| j | Cl | Ph | Ph | 78 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| k | F | Ph | Ph | 71 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
89 |
| l | H | Ph | n-Propyl | 51 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| m | H | Ph | 4-NO2C6H4 | 69 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
The relative configuration of the major diastereomer 3a was determined by NOE measurements (see ESI†), which is in accordance with the absolute configuration of compound 3e determined by X-ray crystal structure analysis (Fig. 2).10
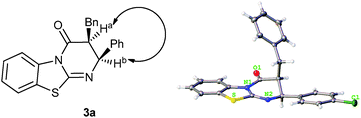 | ||
| Fig. 2 Determination of the relative configuration by NOE (3a) and of the absolute configuration by X-ray crystal structure analysis (3e). | ||
We then tried to extend the substrate scope by employing a 2-indolyl group on the Ar position. In this case, after the Mannich reaction, there are three nucleophilic N-sites for the subsequent lactamization. Interestingly, only the trans-pyrrolo[1,2-a]indolone 3n was obtained via cyclization of the indole N-anion with the acylazolium intermediate with acceptable yield (45%) and excellent stereoselectivity (93% ee, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r.) (Scheme 2).
1 d.r.) (Scheme 2).
With the substrate scope and stereochemical outcome in hand, we propose a plausible catalytic cycle via a stepwise reaction sequence. As shown in Scheme 3, the nucleophilic addition of NHC to α-chloroaldehyde gives rise to the intermediate I, followed by base assisted HCl-elimination to provide the enolate species II. This azolium-enolate then reacts on its Re-face with 2-benzothiazolimine 1via a Mannich reaction to afford the adduct III with cis selectivity. Finally, the benzothiazole N-anion then cyclizes with the acylazolium intermediate liberating the NHC catalyst and producing the desired benzothiazolopyrimidinone 3 in cis configuration.
In conclusion, we have developed an asymmetric NHC-organocatalyzed annulation of 2-benzothiazolimines with α-chloroaldehydes, producing the desired benzothiazolo-pyrimidinones in moderate to good yields with excellent regio- and stereoselectivities. Particularly noteworthy is the reaction of indolyl-bound 2-benzothiazolimine with 2-chloro-3-phenylpropanal. This version of the protocol leads to the formation of pyrrolo[1,2-a]indolone with high regioselectivity and excellent stereoselectivity. Further applications of this protocol in the scope and application are ongoing in our laboratory.
We are grateful for the financial support from the European Research Council (ERC Advanced Grant 320493 “DOMINOCAT”). Q. Ni and X. Song thank the China Scholarship Council for a fellowship.
Notes and references
- (a) R. A. Glennon, J. J. Gaines and M. E. Rogers, J. Med. Chem., 1981, 24, 766–769 CrossRef CAS; (b) R. A. Glennon, S. M. Tejani-Butt, W. Padgett and J. W. Daly, J. Med. Chem., 1984, 27, 1364–1367 CrossRef CAS; (c) A. K. Bhattacharjee, M. G. Hartell, D. A. Nichols, R. P. Hicks, B. Stanton, J. E. van Hamont and W. K. Milhous, Eur. J. Med. Chem., 2004, 39, 59–67 CrossRef CAS PubMed; (d) G. Shukla, A. K. Tiwari, V. K. Singh, A. Bajpai, H. Chandra and A. K. Mishra, Chem. Biol. Drug Des., 2008, 72, 533–539 CrossRef CAS PubMed; (e) M. A. El-Sherbeny, Arzneim. Forsch., 2000, 50, 848–853 CAS.
- (a) V. B. Birman and X. Li, Org. Lett., 2008, 10, 1115–1118 CrossRef CAS PubMed; (b) C. Joannesse, C. P. Johnston, C. Concellón, C. Simal, D. Philp and A. D. Smith, Angew. Chem., Int. Ed., 2009, 48, 8914–8918 CrossRef CAS PubMed; (c) E. R. T. Robinson, C. Fallan, C. Simal, A. M. Z. Slawin and A. D. Smith, Chem. Sci., 2013, 4, 2193–2200 RSC; (d) L. C. Morrill, J. Douglas, T. Lebl, A. M. Z. Slawin, D. J. Fox and A. D. Smith, Chem. Sci., 2013, 4, 4146–4155 RSC; (e) D. G. Stark, L. C. Morrill, P.-P. Yeh, A. M. Z. Slawin, T. J. C. O'Riordan and A. D. Smith, Angew. Chem., Int. Ed., 2013, 52, 11642–11646 CrossRef CAS PubMed; (f) P.-P. Yeh, D. S. B. Daniels, D. B. Cordes, A. M. Z. Slawin and A. D. Smith, Org. Lett., 2014, 16, 964–967 CrossRef CAS PubMed; (g) L. C. Morrill, L. A. Ledingham, J.-P. Couturier, J. Bickel, A. D. Harper, C. Fallan and A. D. Smith, Org. Biomol. Chem., 2014, 12, 624–636 RSC.
- (a) K.-C. Liu and B.-J. Shih, Arch. Pharm., 1985, 318, 276–278 CrossRef CAS; (b) J. Cyrener and K. Burger, Monatsh. Chem., 1995, 126, 319–331 CrossRef CAS; (c) J. M. Mellor and H. Rataj, Tetrahedron Lett., 1996, 37, 2619–2622 CrossRef CAS; (d) R. F. Ambartsumova, Chem. Heterocycl. Compd., 1997, 33, 859–864 CrossRef CAS; (e) A. L. Krasovsky, A. M. Moiseev, V. G. Nenajdenko and E. S. Balenkova, Chem. Heterocycl. Compd., 2004, 40, 667–675 CrossRef; (f) A. B. Atar, Y. S. Jeong and Y. T. Jeong, Tetrahedron, 2014, 70, 5207–5213 CrossRef CAS PubMed.
- For selected reviews, see: (a) D. Enders and T. Balensiefer, Acc. Chem. Res., 2004, 37, 534–541 CrossRef CAS PubMed; (b) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS PubMed; (c) V. Nair, S. Vellalath and B. P. Babu, Chem. Soc. Rev., 2008, 37, 2691–2698 RSC; (d) A. T. Biju, N. Kuhl and F. Glorius, Acc. Chem. Res., 2011, 44, 1182–1195 CrossRef CAS PubMed; (e) J. Izquierdo, G. E. Hutson, D. T. Cohen and K. A. Scheidt, Angew. Chem., Int. Ed., 2012, 51, 11686–11698 CrossRef CAS PubMed; (f) A. Grossmann and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 314–325 CrossRef CAS PubMed; (g) X. Bugaut and F. Glorius, Chem. Soc. Rev., 2012, 41, 3511–3522 RSC; (h) H. U. Vora, P. Wheeler and T. Rovis, Adv. Synth. Catal., 2012, 354, 1617–1639 CrossRef CAS PubMed; (i) S. J. Ryan, L. Candish and D. W. Lupton, Chem. Soc. Rev., 2013, 42, 4906–4917 RSC; (j) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS PubMed.
- (a) C. Burstein and F. Glorius, Angew. Chem., Int. Ed., 2004, 43, 6205–6208 CrossRef CAS PubMed; (b) S. S. Sohn, E. L. Rosen and J. W. Bode, J. Am. Chem. Soc., 2004, 126, 14370–14371 CrossRef CAS PubMed.
- For examples employing NHC-derived enolates or homoenolates, see: (a) B. Cardinal-David, D. E. A. Raup and K. A. Scheidt, J. Am. Chem. Soc., 2010, 132, 5345–5347 CrossRef CAS PubMed; (b) T.-Y. Jian, P.-L. Shao and S. Ye, Chem. Commun., 2011, 47, 2381–2383 RSC; (c) T.-Y. Jian, L.-H. Sun and S. Ye, Chem. Commun., 2012, 48, 10907–10909 RSC; (d) J. Dugal-Tessier, E. A. O'Bryan, T. B. H. Schroeder, D. T. Cohen and K. A. Scheidt, Angew. Chem., Int. Ed., 2012, 51, 4963–4967 CrossRef CAS PubMed; (e) X. Zhao, K. E. Ruhl and T. Rovis, Angew. Chem., Int. Ed., 2012, 51, 12330–12333 CrossRef CAS PubMed; (f) J. Mo, R. Yang, X. Chen, B. Tiwari and Y. R. Chi, Org. Lett., 2012, 15, 50–53 CrossRef PubMed; (g) H. Lv, B. Tiwari, J. Mo, C. Xing and Y. R. Chi, Org. Lett., 2012, 14, 5412–5415 CrossRef CAS PubMed; (h) J. Izquierdo, A. Orue and K. A. Scheidt, J. Am. Chem. Soc., 2013, 135, 10634–10637 CrossRef CAS PubMed; (i) X. Chen, X. Fang and Y. R. Chi, Chem. Sci., 2013, 4, 2613–2618 RSC; (j) E. O. B. McCusker and K. A. Scheidt, Angew. Chem., Int. Ed., 2013, 52, 13616–13620 CrossRef PubMed; (k) Q. Ni, H. Zhang, A. Grossmann, C. C. J. Loh, C. Merkens and D. Enders, Angew. Chem., Int. Ed., 2013, 52, 13562–13566 CrossRef CAS PubMed; (l) C. Guo, M. Schedler, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2014, 53, 10232–10236 CrossRef CAS PubMed; (m) J.-L. Li, B. Sahoo, C.-G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2014, 53, 10515–10519 CrossRef CAS PubMed; (n) M. Wang, Z.-Q. Rong and Y. Zhao, Chem. Commun., 2014, 50, 15309–15312 RSC.
- N. Duguet, C. D. Campbell, A. M. Z. Slawin and A. D. Smith, Org. Biomol. Chem., 2008, 6, 1108–1113 CAS.
- Y.-R. Zhang, L. He, X. Wu, P.-L. Shao and S. Ye, Org. Lett., 2008, 10, 277–280 CrossRef CAS PubMed.
- H.-M. Zhang, Z.-H. Gao and S. Ye, Org. Lett., 2014, 16, 3079–3081 CrossRef CAS PubMed.
- CCDC 1029497 contains the supplementary crystallographic data for compound 3e reported in this communication.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1029497. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc08594a |
| This journal is © The Royal Society of Chemistry 2015 |