 Open Access Article
Open Access ArticleNi-Catalyzed α-arylation of esters and amides with phenol derivatives†
Eva
Koch‡
a,
Ryosuke
Takise‡
b,
Armido
Studer
a,
Junichiro
Yamaguchi
*b and
Kenichiro
Itami
*bc
aOrganisch-Chemisches Institut, Westfälische Wilhelms-Universität, Corrensstraße 40, 48149 Münster, Germany
bInstitute of Transformative Bio-Molecules (WPI-ITbM) and Department of Chemistry, Graduate School of Science, Nagoya University, Chikusa, Nagoya 464-8602, Japan. E-mail: junichiro@chem.nagoya-u.ac.jp; itami@chem.nagoya-u.ac.jp
cJST, ERATO, Itami Molecular Nanocarbon Project, Nagoya University, Chikusa, Nagoya 464-8602, Japan
First published on 21st November 2014
Abstract
A nickel-catalyzed α-arylation of esters and amides with phenol derivatives has been accomplished. In the presence of our unique nickel catalyst, prepared in situ from Ni(cod)2, 3,4-bis(dicyclohexylphosphino)thiophene (dcypt), and K3PO4, various esters and amides undergo α-arylation with O-arylpivalates or O-arylcarbamates to afford the corresponding coupling products. The thus obtained α-aryl esters and amides are useful precursors of privileged motifs such as α-arylcarboxylic acids and β-arylamines.
α-Aryl esters and amides are useful intermediates in organic synthesis that can be converted into prevalent motifs such as α-arylcarboxylic acids and β-arylamines.1 Therefore, over the last two decades, significant efforts have been devoted to develop concise, direct, and environmental friendly methods constructing these privileged motifs. A representative method for the synthesis of α-aryl esters and amides is the Pd-catalyzed α-arylation of esters and amides with (pseudo)haloarenes. A number of reaction conditions have been reported by several groups including Miura, Hartwig and Buchwald, who are pioneers in this field.2,3 However, these state-of-the-art α-arylation reactions typically necessitate expensive Pd catalysts as well as haloarenes as arylating agents.4,5 Herein, we report that our unique nickel catalyst allows the α-arylation of esters and amides with phenol derivatives as arylating agents and K3PO4 as a mild base.
In recent years, phenol derivatives have received much attention as green and inexpensive arylating agents via C–O activation (alternative to haloarenes) in coupling chemistry.6,7 Our group has also contributed to this field by developing unique nickel catalysts that can activate not only the phenolic C–O bonds but also the C–H bonds of counter coupling components to achieve otherwise difficult C–H/C–O activation/coupling processes. For example, in the presence of a nickel catalyst prepared from Ni(cod)2 (cod = 1,5-cyclohexadiene) and 1,2-bis(dicyclohexylphosphino)ethane (dcype), the C–H/C–O type coupling of 1,3-azoles and phenol derivatives can be realized.8
Very recently, we translated the established nickel-based C–H/C–O activation mode to α-arylation of ketones with phenol derivatives (O-arylpivalates or O-arylcarbamates), which addresses some of the drawbacks in the state-of-the-art α-arylation chemistry (Scheme 1).9 During this study, we found that the newly developed ligand, 3,4-bis(dicyclohexylphosphino)thiophene (dcypt), is superior to our first-generation ligand dcype. Encouraged by the facts that our new ligand not only displays high reactivity in activating C–H and C–O bonds but is also stable in air, we wondered whether the Ni(cod)2/dcypt catalyst can be applied to a more challenging class of substrates, esters and amides, in α-arylation chemistry.
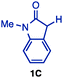
We began by investigating the coupling reaction of representative carbonyl compounds (ketone 1A, ester 1B, and amide 1C) and phenol derivatives (Ar–OR) in the presence of a Ni(cod)2/dcypt catalyst (Table 1). In order to study the trend in reactivity, carbonyl compounds (1A–1C; 1.5 equiv.) were treated with Ar–OR (2; 1.0 equiv.) in toluene at 150 °C for 24 h, in the presence of Ni(cod)2 (10 mol%), dcypt (20 mol%), and K3PO4 (1.5 equiv.). Ketone 1A was coupled with naphthalen-2-yl pivalate (“pivalate”) and naphthalen-2-yl dimethylcarbamate (“carbamate”) to afford the corresponding product 3A in 91% and 77% isolated yields, respectively (entries 1 and 2).9 When the pivalate was changed to i-butyrate, the yield of 3A decreased (31% yield, entry 3), and a further change to acetate shuts down the reaction. In the case of the reactions using carbonate, tosylate, and phosphate, product 3A was formed in moderate yields (entries 4–6). Switching from a ketone to an ester system, when ester 1B (2.0 equiv.) was reacted with pivalate, the coupling product 3B was produced only in 11% yield (entry 7). Surprisingly, when 1B was reacted with aryl carbamate, 3B was produced in good yield (63% NMR yield and 47% isolated yield, entry 8). In contrast, i-butyrate, carbonate, and tosylate gave inferior results (entries 9–12).
| Entry | Carbonyls | Ar–OR | Yield of 3b/% |
|---|---|---|---|
| a Conditions: 1 (0.45 mmol), 2 (0.3 mmol), Ni(cod)2 (0.03 mmol), dcypt (0.06 mmol), K3PO4 (0,45 mmol), toluene (1.2 mL), 150 °C, 24 h. b NMR yield. c Isolated yield. d 1B (0.60 mmol) was used. e Ni(cod)2 (0.015 mmol) and dcypt (0.015 mmol) were used. | |||
| 1 |

|
Pivalate | 98 (91)c |
| 2 | Carbamate | (77)c | |
| 3e | i-Butyrate | 31 | |
| 4 | Carbonate | 49 | |
| 5 | Tosylate | 43 | |
| 6 | Phosphate | 49 | |
| 7d |

|
Pivalate | 11 |
| 8d | Carbamate | 63 (47)c | |
| 9d,e | i-Butyrate | 7 | |
| 10d | Carbonate | 46 | |
| 11d | Tosylate | 24 | |
| 12d,e | Phosphate | 36 | |
| 13e |
|
Pivalate | (76)c |
| 14 | Carbamate | 76 | |
| 15 | i-Butyrate | 9 | |
| 16 | Carbonate | — | |
| 17 | Tosylate | 40 | |
| 18e | Phosphate | 24 | |

|
|||
When amide 1C was used as a carbonyl substrate, both pivalate and carbamate reacted smoothly (entries 13 and 14), whereas other arylating reagents were much less reactive (entries 15–18). Although we further tried to optimize other parameters such as ligands, bases, temperature, and solvents, we did not get significantly superior conditions.10 Thus, with an appropriate choice of arylation agents, the α-arylation of esters and amides with phenol derivatives can now be realized by a Ni(cod)2/dcypt catalyst. Aryl carbamate is a general arylating agent applicable to all of the tested carbonyls and aryl pivalate is a somewhat better reagent in the case of amide arylation.
Next, we examined the coupling reactions of various esters and O-arylcarbamates 2 (Scheme 2). When the aryl group on the α-position of the ester was changed from p-methoxyphenyl to o-fluorophenyl, o-trifluoromethylphenyl, and phenyl, the corresponding products 3D–3I were obtained in moderate to good yields.11 Functional groups such as ester (in 3G) and the basic nitrogen atom (in 3H) were tolerated well under the present coupling conditions. However, the reactions of simple phenol derivatives, instead of naphthol derivatives, gave the corresponding coupling product in low yield. For example, 1B was coupled with m-tolyl dimethylcarbamate to afford the product 3I in 27% yield. The intermediacy of the Meisenheimer complex or η2-coordination perhaps accounts for the higher reactivity of naphthyl pivalates vs. aryl pivalates.7e
We also investigated the α-arylation of amides with O-arylpivalates (Scheme 3). In addition to naphthol-based pivalates, phenol derivatives can also be used as arylating agents, as exemplified by the synthesis of 3K and 3L. The use of electron-rich phenol derivatives resulted in low yield (for example, 3M). Last, but not least, our preliminary substrate screening identified that, in addition to oxindole derivatives, succinimide and thioamide derivatives can also be arylated under the influence of the Ni(cod)2/dcypt catalyst to give the coupling products (3O and 3P).11
The present study not only shows the broad applicability of our unique nickel catalyst (Ni-dcypt) in C–H/C–O activation/coupling processes, but also represents the first demonstration of Ni-catalyzed α-arylation of esters and amides with O-arylpivalates or O-arylcarbamates. Various synthetically useful α-aryl esters and amides can now be synthesized from non-halogenated arylating agents through the agency of Ni-dcypt catalysis. Mechanistic studies as well as further modifications of the nickel catalyst to achieve a broader scope for both the carbonyl and phenol derivatives are ongoing in our laboratory.
This work was supported by the Funding Program for Next Generation World-Leading Researchers from JSPS (220GR049 to K.I.), a Grant-in-Aid for Scientific Research on Innovative Areas “Molecular Activation Directed toward Straightforward Synthesis” (25105720 to J.Y.), and KAKENHI (25708005 to J.Y.) from MEXT. E.K. thanks the International Research Training Group Münster/Nagoya for support. ITbM is supported by the World Premier International Research Center (WPI) Initiative, Japan.
Notes and references
- For selected reviews and accounts, see: (a) F. Bellina and R. Rossi, Chem. Rev., 2010, 110, 1082 CrossRef CAS PubMed; (b) C. C. C. Johansson and T. J. Colacot, Angew. Chem., Int. Ed., 2010, 49, 676 CrossRef CAS PubMed; (c) P. Noväk and R. Martin, Curr. Org. Chem., 2011, 15, 3233 CrossRef; (d) D. A. Culkin and J. F. Hartwig, Acc. Chem. Res., 2003, 36, 234 CrossRef CAS PubMed.
- For representative reports on Pd-catalyzed α-arylation of esters with haloarenes, see: (a) T. Satoh, J. Inoh, Y. Kawamura, Y. Kawamura, M. Miura and M. Nomura, Bull. Chem. Soc. Jpn., 1998, 71, 2239 CrossRef CAS; (b) W. A. Moradi and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 7996 CrossRef CAS PubMed; (c) S. Lee, N. A. Beare and J. F. Hartwig, J. Am. Chem. Soc., 2001, 123, 8410 CrossRef CAS; (d) M. Jørgensen, S. Lee, X. Liu, J. P. Wolkowski and J. F. Hartwig, J. Am. Chem. Soc., 2002, 124, 12557 CrossRef PubMed; (e) O. Gaertzen and S. L. Buchwald, J. Org. Chem., 2002, 67, 465 CrossRef CAS PubMed; (f) N. A. Beare and J. F. Hartwig, J. Org. Chem., 2002, 67, 541 CrossRef CAS PubMed; (g) D. Sole and O. Serrano, J. Org. Chem., 2008, 73, 2476 CrossRef CAS PubMed; (h) E. A. Bercot, S. Caille, T. M. Bostick, K. Ranganathan, R. Jensen and M. M. Faul, Org. Lett., 2008, 10, 5251 CrossRef CAS PubMed; (i) T. Hama and J. F. Hartwig, Org. Lett., 2008, 10, 1545 CrossRef CAS PubMed; (j) T. Hama and J. F. Hartwig, Org. Lett., 2008, 10, 1549 CrossRef CAS PubMed; (k) L. Jiang, S. Weist and S. Jansat, Org. Lett., 2009, 11, 1543 CrossRef CAS PubMed.
- For representative reports on Pd-catalyzed α-arylation of amides with haloarenes, see: (a) K. H. Shaughnessy, B. C. Hamann and J. F. Hartwig, J. Org. Chem., 1998, 63, 6546 CrossRef CAS; (b) S. Lee and J. F. Hartwig, J. Org. Chem., 2001, 66, 3402 CrossRef CAS PubMed; (c) T. Hama, D. A. Culkin and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 4976 CrossRef CAS PubMed; (d) Y.-X. Jia, J. M. Hillgren, E. L. Watson, S. P. Marsdenb and E. P. Kündig, Chem. Commun., 2008, 4040 RSC; (e) M. J. Durbin and M. C. Willis, Org. Lett., 2008, 10, 1413 CrossRef CAS PubMed; (f) A. M. Taylor, R. A. Altman and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 9900 CrossRef CAS PubMed; (g) L. Ackermann, R. Vicente and N. Hofmann, Org. Lett., 2009, 11, 4274 CrossRef CAS PubMed; (h) B. Zheng, T. Jia and P. J. Walsh, Adv. Synth. Catal., 2014, 356, 165 CrossRef CAS PubMed.
- Few α-arylation of esters and amides under non-Pd systems, see: (a) D. J. Spielvogel and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 3500 CrossRef CAS PubMed; (b) S. F. Yip, H. Y. Cheung, Z. Zhou and F. Y. Kwong, Org. Lett., 2007, 9, 3469 CrossRef CAS PubMed; (c) B. Peng, D. Geerdink, C. Farés and N. Maulide, Angew. Chem., Int. Ed., 2014, 53, 5462 CrossRef CAS PubMed.
- Only one example of Pd-catalyzed α-arylation of esters with phenol derivatives (aryl tosylates) was reported. H. N. Nguyen, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2003, 125, 11818 CrossRef CAS PubMed.
- For reviews, see (a) J. Yamaguchi, K. Muto and K. Itami, Eur. J. Org. Chem., 2013, 19 CrossRef CAS; (b) S. Z. Tasker, E. A. Standley and T. F. Jamison, Nature, 2014, 509, 299 CrossRef CAS PubMed; (c) J. Cornella, C. Zarate and R. Martin, Chem. Soc. Rev., 2014, 43, 8081 RSC; (d) B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg and V. Percec, Chem. Rev., 2011, 111, 1346 CrossRef CAS PubMed.
- For selected recent examples of C–O activation, see: (a) M. Tobisu, T. Shimasaki and N. Chatani, Angew. Chem., Int. Ed., 2008, 47, 4866 CrossRef CAS PubMed; (b) D.-G. Yu and Z.-J. Shi, Angew. Chem., Int. Ed., 2011, 50, 7097 CrossRef CAS PubMed; (c) K. W. Quasdorf, A. Antoft-Finch, P. Liu, A. L. Silberstein, A. Komaromi, T. Blackburn, S. D. Ramgren, K. N. Houk, V. Snieckus and N. K. Garg, J. Am. Chem. Soc., 2011, 133, 6352 CrossRef CAS PubMed; (d) C. Zarate and R. Martin, J. Am. Chem. Soc., 2014, 136, 2236 CrossRef CAS PubMed; (e) A. Correa, T. León and R. Martin, J. Am. Chem. Soc., 2014, 136, 1062 CrossRef CAS PubMed.
- (a) K. Muto, J. Yamaguchi and K. Itami, J. Am. Chem. Soc., 2012, 134, 169 CrossRef CAS PubMed; (b) K. Amaike, K. Muto, J. Yamaguchi and K. Itami, J. Am. Chem. Soc., 2012, 134, 13573 CrossRef CAS PubMed; (c) L. Meng, Y. Kamada, K. Muto, J. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2013, 52, 10048 CrossRef CAS PubMed; (d) K. Muto, J. Yamaguchi, A. Lei and K. Itami, J. Am. Chem. Soc., 2013, 135, 16384 CrossRef CAS PubMed; (e) H. Xu, K. Muto, J. Yamaguchi, C. Zhao, K. Itami and D. G. Musaev, J. Am. Chem. Soc., 2014, 136, 14834 CrossRef CAS PubMed.
- R. Takise, K. Muto, J. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2014, 53, 6791 CrossRef CAS PubMed.
- See the ESI† for details.
- The reactions gave mono-arylation products as main products, but we also detected small amounts of diarylation products in the reaction mixture.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, and 1H and 13C NMR spectra of products. See DOI: 10.1039/c4cc08426h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |




