Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Single-shot titrations and reaction monitoring by slice-selective NMR spectroscopy†
T.
Niklas
,
D.
Stalke
and
M.
John
*
Institut für Anorganische Chemie, Georg-August-Universität Göttingen, Tammannstraße 4, 37077 Göttingen, Germany. E-mail: mjohn@gwdg.de
First published on 28th November 2014
Abstract
A new method, based on slice-selective NMR spectroscopy of inhomogeneous mixtures, is introduced to perform NMR titrations and reaction monitoring in a single experiment. The method was applied to the titration of a lithium salt with 12-crown-4, and to the reaction of nBuLi with N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDTA).
NMR spectroscopy plays an increasingly important role in the elucidation of structural and dynamic features of inorganic, organic and biomolecular compounds and their interactions. For example, chemical shift titration is a powerful method to determine the stoichiometry and stability constants of complexes in coordination, supramolecular and medicinal chemistry.1–3 In such an experiment, the chemical shift of a particular resonance attributed to one component (e.g. the metal, host or target) is monitored in a series of NMR spectra, while the concentration of the other component (e.g. the guest or ligand) is systematically varied. In a different approach, NMR is applied to investigate chemical reactions by monitoring resonances of substrates and products in a series of individual NMR spectra over time. The observation of fast reactions and short-lived intermediates4,5 has become possible through custom build NMR hardware and techniques such as stopped flow6,7 and rapid-injection NMR.8–12
In our research group slice-selective NMR spectroscopy has lately become an important technique to study diffusion of solvents and solutes into polymers.13 Using standard solution NMR instrumentation, slice-selection is accomplished by shaped radiofrequency pulses with variable frequency offsets in the presence of a magnetic field gradient along the axis of the NMR tube.13–17 For highly sensitive nuclei such as 1H, 7Li, 19F or 31P, a series of typically ∼20 conventional NMR spectra of individual horizontal (∼1 mm) slices within the active sample volume (∼2 cm) is obtained in less than 2 min. Here we propose a fast chemical shift titration method based on slice-selective NMR spectroscopy in combination with a concentration gradient of the ligand component rather than incrementing the concentration step-by-step. Alternatively, chemical reactions between two substrates diffusing towards each other may be monitored.
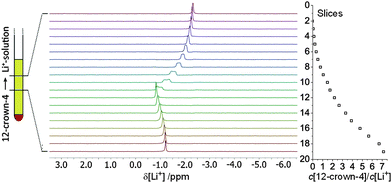
As a case study for the slice-selective titration method we investigated the complexation of a 7Li ion with 12-crown-4. For this purpose, 12-crown-4 (50 μL, 3.1 mmol, m.p. 16 °C) was filled into a standard 5 mm NMR tube and cooled to 5 °C. Then a solution of LiClO4 (24 mg, 2.3 mmol) in acetonitrile-d3 (0.45 mL) was layered on top of the solid ether. This procedure prevents initial mixing of both components prior to the measurements. Inside the NMR magnet (25 °C, standing tube) the ether melts and slowly diffuses into the LiClO4 solution resulting in a smooth concentration gradient along the tube axis. Diffusion of LiClO4 into the ether phase occurs likewise, but with little impact on the 7Li concentration due to the 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio. Likewise, the impact of molecular diffusion during the gradient pulse is negligible. Approximately 3, 6 and 9 h after sample preparation slice-selective 1H and 7Li NMR measurements were performed (see ref. 13 and ESI† for details).13 For each slice, absolute integrals of both, the 7Li resonance of LiClO4 and the 1H resonance of 12-crown-4, were measured and converted into concentrations using homogenous reference samples. This way, the 7Li chemical shift observed in each slice can be assigned to a specific ether/lithium ratio.
1 volume ratio. Likewise, the impact of molecular diffusion during the gradient pulse is negligible. Approximately 3, 6 and 9 h after sample preparation slice-selective 1H and 7Li NMR measurements were performed (see ref. 13 and ESI† for details).13 For each slice, absolute integrals of both, the 7Li resonance of LiClO4 and the 1H resonance of 12-crown-4, were measured and converted into concentrations using homogenous reference samples. This way, the 7Li chemical shift observed in each slice can be assigned to a specific ether/lithium ratio.
Fig. 1 shows the series of 19 slice-selective 7Li NMR spectra recorded after 6 h (for spectra recorded after 3 and 9 h see ESI†). Additional slice-selective 1H spectra (see also ESI†) confirm that 12-crown-4 diffuses from the bottom to the top and builds up a smooth gradient, while the LiClO4 concentration is approximately constant. Slice 1 (at the top of the active volume) shows a narrow 7Li resonance at −2.3 ppm, typical for Li+ coordinated by four acetonitrile molecules. As the ether concentration increases, the 7Li resonance is shifted downfield and reaches a maximum (−0.8 ppm) in slice 12, where the 12-crown-4 and LiClO4 concentrations are approximately equal and thus the [Li(12-crown-4)]+ complex dominates.18 In the presence of an excess of 12-crown-4, the 7Li resonance is then shifted again upfield until it reaches −1.2 ppm in slice 19 (7-fold excess, [Li(12-crown-4)2]+). The resulting titration curve (see ESI†) fully agrees with previous reports. Note that the 7Li resonances in slices 6 to 11 are increasingly broadened due to (rectangular weighted) summation over the ether concentration gradient within each slice. This broadening could be overcome by further decreasing the slice width, thereby increasing the spatial resolution along the tube axis, but at the cost of reduced sensitivity, which may render weaker signals undetectable.18,19
The application of slice-selective NMR spectroscopy to reaction monitoring is demonstrated with the reaction between nBuLi and PMDTA. Due to the high reactivity of organolithium reagents and to prevent rapid convection and/or diffusion out of the active sample volume, nBuLi was first diffused into a polystyrene gel matrix, and all steps of the preparation were carried out strictly under argon atmosphere. For this purpose, a solution of nBuLi in n-hexane (0.4 mL, 1.9 M, 0.74 mmol) was concentrated in vacuo and toluene-d8 (0.40 mL) was added. This solution was then transferred into a 5 mm NMR tube, and a cylindrical polystyrene stick (10 × 3.8 mm, crosslinked through 0.2 vol% divinylbenzene, for preparation see ref. 20) was immersed in the solution approximately 1 cm above the bottom of the tube. After 7 days (the polystyrene stick had swollen to a length of ∼3 cm) the supernatant nBuLi–toluene solution above the polymer was removed and replaced by a solution of PMDTA (0.12 mL, 0.60 mmol) in toluene-d8 (0.15 mL). Slice-selective 1H and 7Li NMR measurements started after ∼3 h and went on for three days.
Fig. 2 (bottom left) shows the slice-selective 7Li NMR spectra three days after the addition of PMDTA to the polymer imbibed with nBuLi (for spectra recorded after 3 h, 1 and 2 days, see ESI†). Slices 19 and 18 at the bottom of the active volume still show a signal for unreacted nBuLi at 2.3 ppm, in agreement with the absence of 1H signals of PMDTA in these slices (see also ESI†). On the other hand, in slices 1–12 a narrow resonance at 1.1 ppm is observed, which was assigned to the product of the reaction, lithiated PMDTA (see Fig. 2 top). Satellite peaks in slices 6–12 arise from residual quadrupolar couplings (RQCs) due to partial orientation of molecules inside the stretched gel.20 The size of the RQCs varies with the amount of strain and is not constant over the gel body unless the gel is equilibrated for a prolonged period of time.13,15 Slices 1 to 5 are located outside the polymer and hence only a singlet is observed for lithiated PMDTA in this region.
Increasingly broad signals in slices 17–13 with chemical shifts between 2.3 and 1.6 ppm mark the reaction front between PMDTA and nBuLi, at which multiple dynamic processes take place.21 Although the corresponding 1H spectra are relatively crowded with signals of different reaction components as well as residual signals from the polymer, the isolated region of the nBuLi α-CH2 protons (−0.5 to −1 ppm) is quite informative (Fig. 2 bottom right): the signal for the nBuLi hexamer22,23 at −0.92 ppm moves downfield and broadens from slice 19 to 13, presumably due to unspecific deaggregation and/or complexation of nBuLi by PMDTA. These processes are fast to intermediate on the NMR timescale such that only averaged chemical shifts are observed by 1H and 7Li NMR. From slice 12 onwards, the α-CH2 region is free of signals indicating that nBuLi has completely reacted to butane.
The most remarkable feature in slices 18–13 is the appearance of an additional, unshifted 1H resonance at −0.59 ppm with a maximum intensity in slice 15. This signal was assigned to the complex [(nBuLi)2PMDTA]2 (see Fig. 2 top), which seems to be remarkably stable and has been characterised by X-ray crystallography, 1H NMR and quantum chemical calculations as an intermediate in the lithiation of PMDTA before.21 Further 1H signals reported for [(nBuLi)2PMDTA]2 were found in the crowded regions of the corresponding slices (see ESI†).
The connection between the reaction progress and the appearance of the peak at −0.59 ppm can nicely be illustrated by integrating this peak and the remaining nBuLi α-CH2 signal over the course of three days. Fig. 3 illustrates the motion of the reaction front, where the nBuLi α-CH2 signal (orange) vanishes and the signal of the corresponding CH2-moiety within the [(nBuLi)2PMDTA]2 complex (green) appears. The latter builds up and decays exclusively at the reaction front, indicating its intermediate character. While 3 h after the addition the front is relatively sharp with little formation of [(nBuLi)2PMDTA]2, it becomes significantly blurred as it moves downwards with time. This is in accordance with what would be expected from diffusion in a gel.24 The dip in the nBuLi concentration which is initially observed in the centre of the gel most likely arises from slow and incomplete diffusion of nBuLi into the polymer during sample preparation.13 This again underlines that the nBuLi hexamer (Mw = 383 g mol−1) may be regarded as rather static compared to the PMDTA molecules (Mw = 173 g mol−1). No separate 7Li NMR signal was found for [(nBuLi)2PMDTA]2, most likely due to fast dynamic exchange with the signal of nBuLi. Likewise, no evidence was found for further breaking down of [(nBuLi)2PMDTA]2 into smaller units representing active species/intermediates during the lithiation of PMDTA. Note that a monomeric nBuLi·PMDTA adduct has been proposed but its existence has not yet been experimentally confirmed.25,26
In the present study we could show that slice-selective NMR spectroscopy is a simple method to perform single-shot NMR titrations and in situ observation of reactions, using a routine NMR instrument. The “fast titration” was successfully tested in the complexation of a lithium salt by 12-crown-4, where it was able to reproduce a conventional 7Li chemical shift titration curve. Reaction monitoring by slice-selective NMR was tested in the reaction between PMDTA and nBuLi, where the previously characterised intermediate [(nBuLi)2PMDTA]2 could be identified. A stretched polystyrene gel was used as medium (i) to slow down the reaction and avoid convection, (ii) to immobilise one reactant (nBuLi) with respect to the other (PMDTA) and (iii) to principally enable also the observation of 7Li RQCs as additional source of structural information. Polystyrene is chemically inert, tolerates highly reactive reagents,20 and is swollen by a broad range of solvents.27 A further advantage of the gel method is the possibility to adjust the slope of the reaction front and hence to “zoom” into the interesting region. It should therefore be possible to apply slice-selective NMR to a broad range of reactions to obtain information about mechanisms as well as stoichiometry of complexes and products.
T. N. thanks the Studienstiftung des Deutschen Volkes for financial support.
Notes and references
- M. J. Hynes, J. Chem. Soc., Dalton Trans., 1993, 311–312 RSC.
- I. W. Wyman and D. H. Macartney, Org. Biomol. Chem., 2008, 6, 1796–1801 CAS.
- J. S. Renny, L. L. Tomasevich, E. H. Tallmadge and D. B. Collum, Angew. Chem., Int. Ed., 2013, 52, 11998–12013 CrossRef CAS PubMed.
- A.-C. Pöppler, M. M. Meinholz, H. Faßhuber, A. Lange, M. John and D. Stalke, Organometallics, 2011, 31, 42–45 CrossRef.
- A.-C. Pöppler, M. Granitzka, R. Herbst-Irmer, Y.-S. Chen, B. B. Iversen, M. John, R. A. Mata and D. Stalke, Angew. Chem., Int. Ed., 2014, 53, 13282–13287 CrossRef PubMed.
- P. J. Hore, S. L. Winder, C. H. Roberts and C. M. Dobson, J. Am. Chem. Soc., 1997, 119, 5049–5050 CrossRef CAS.
- M. D. Christianson, E. H. P. Tan and C. R. Landis, J. Am. Chem. Soc., 2010, 132, 11461–11463 CrossRef CAS PubMed.
- J. F. McGarrity and J. Prodolliet, J. Org. Chem., 1984, 49, 4465–4470 CrossRef CAS.
- J. F. McGarrity, C. A. Ogle, Z. Brich and H. R. Loosli, J. Am. Chem. Soc., 1985, 107, 1810–1815 CrossRef CAS.
- W. Bauer, M. Feigel, G. Mueller and P. v. R. Schleyer, J. Am. Chem. Soc., 1988, 110, 6033–6046 CrossRef CAS PubMed.
- S. H. Bertz, S. Cope, M. Murphy, C. A. Ogle and B. J. Taylor, J. Am. Chem. Soc., 2007, 129, 7208–7209 CrossRef CAS PubMed.
- S. E. Denmark, B. M. Eklov, P. J. Yao and M. D. Eastgate, J. Am. Chem. Soc., 2009, 131, 11770–11787 CrossRef CAS PubMed.
- A.-C. Pöppler, S. Frischkorn, D. Stalke and M. John, ChemPhysChem, 2013, 14, 3103–3107 CrossRef PubMed.
- R. Freeman, Concepts Magn. Reson., Part A, 2011, 38, 1–6 CrossRef.
- P. Trigo-Mouriño, C. Merle, M. R. M. Koos, B. Luy and R. R. Gil, Chem. – Eur. J., 2013, 19, 7013–7019 CrossRef PubMed.
- G. E. Wagner, P. Sakhaii, W. Bermel and K. Zangger, Chem. Commun., 2013, 49, 3155–3157 RSC.
- B. Sathyamoorthy, D. M. Parish, G. T. Montelione, R. Xiao and T. Szyperski, ChemPhysChem, 2014, 15, 1872–1879 CrossRef CAS PubMed.
- M. C. Masiker, C. L. Mayne, B. J. Boone, A. M. Orendt and E. M. Eyring, Magn. Reson. Chem., 2010, 48, 94–100 CAS.
- M. C. Masiker, C. L. Mayne and E. M. Eyring, Magn. Reson. Chem., 2006, 44, 220–229 CrossRef CAS PubMed.
- A.-C. Pöppler, H. Keil, D. Stalke and M. John, Angew. Chem., Int. Ed., 2012, 51, 7843–7846 CrossRef PubMed.
- C. Strohmann and V. H. Gessner, Angew. Chem., Int. Ed., 2007, 46, 4566–4569 CrossRef CAS PubMed.
- D. Margerison and J. P. Newport, Trans. Faraday Soc., 1963, 59, 2058–2063 RSC.
- T. Kottke and D. Stalke, Angew. Chem., Int. Ed., 1993, 32, 580–582 CrossRef.
- S. C. George and S. Thomas, Prog. Polym. Sci., 2001, 26, 985–1017 CrossRef CAS.
- C. Strohmann, T. Seibel and K. Strohfeldt, Angew. Chem., Int. Ed., 2003, 42, 4531–4533 CrossRef CAS PubMed.
- V. H. Gessner, C. Däschlein and C. Strohmann, Chem. – Eur. J., 2009, 15, 3320–3334 CrossRef CAS PubMed.
- C. M. Thiele, Eur. J. Org. Chem., 2008, 5673–5685 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed procedures, spectra, additional schemes. See DOI: 10.1039/c4cc08329f |
| This journal is © The Royal Society of Chemistry 2015 |