Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Circular serendipity: in situ ligand transformation for the self-assembly of an hexadecametallic [CuII16] wheel†
Andreas K.
Kostopoulos
a,
Athanassios D.
Katsenis
a,
Jamie M.
Frost
b,
Vadim G.
Kessler
c,
Euan K.
Brechin
*b and
Giannis S.
Papaefstathiou
*a
aLaboratory of Inorganic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis, 157 71 Zografou, Greece. E-mail: gspapaef@chem.uoa.gr; Fax: +30 210 727 4782; Tel: +30 210 727 4840
bEaStCHEM School of Chemistry, The University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ, UK. E-mail: ebrechin@staffmail.ed.ac.uk; Fax: +44 (0)11 275 4598; Tel: +44 (0)131 650 7545
cDepartment of Chemistry, Swedish University of Agricultural Sciences, Box 7015, 750 07 Uppsala, Sweden
First published on 15th October 2014
Abstract
A [CuII16] wheel was isolated serendipitously from the reaction of acetylacetone dioxime with copper(II) chloride and lanthanide ions in a reaction initially designed to produce heterometallic 3d–4f cages. The ligand has been transformed in situ to three different forms, all found within the [Cu16] wheel, with the original ligand completely absent.
Synthetic methodologies for the construction of polymetallic cages of paramagnetic metal ions have (rightly) always spanned the entire spectrum from serendipity to designed assembly.1 There is clear method in the madness of the former approach: the choice of metal dictates the nature of the magnetic properties of the resulting cluster, and careful thought is exercised in ligand design so that metal ions of a particular type, oxidation state and geometry can be linked in a particular fashion. It is the flexibility in the coordination of both metal and ligand, and the presence of (templating) hydroxide or oxide ions, that renders absolute structure prediction difficult, even if the building blocks – the small fragments dictating topology – are known. Perhaps it is this serendipity that often sees beautiful and novel structural types remaining unreferenced by those outside the community, but the sheer variety and aesthetically pleasing nature of these molecules – beyond the imagination of the humble scientist – is undeniable and reason enough to justify the approach. Such examples include, but are not limited to, the spectacular [Mn84] torus,2 the [Mn32] double-decker wheel,3 the [Fe17] all-ferric analogue of magnetite obtained from the simple dissolution of FeBr3 in wet pyridine,4 the chiral [Er60]5 stabilized by eight μ6-CO32− ions derived from ligand decomposition, the giant [Cu17Mn28]6 cage comprising six formates derived from the hydrolysis of the solvent (DMF), the enormous [Fe64]7 and [Fe168]8 cubic-shaped cages, the [Fe30] icosidodecahedron encapsulated inside a {MoVI72FeIII30} POM – a finite-size version of a Kagomé lattice,9 and the [Na4Mn40] and [Mn44] loop-of-loops.10 Many in the molecular magnetism community would also argue that the most ‘magnetically interesting’ complexes of recent times also have their origins in serendipity. The structures of the molecular magnets Mn12, Fe8, Fe4, Ni4, Mn4, Mn6, and Cr7M families (to name but a few) for example, could not have been predicted, but thorough exploitation thereafter has seen many fascinating physical properties uncovered and exploited.11
The level of structural control over reaction product(s) is decreased yet further when the ligand(s) undergo(es) in situ metal-assisted transformation(s). Di-2-pyridyl ketone (py2CO), for example, is known to undergo metal-assisted transformations, with more than ten different forms of py2CO having been identified.12 In some cases, two different forms of py2CO have been found within the same cluster.13 Indeed there are a number of polynuclear metal complexes comprising two different forms of a ligand obtained by in situ metal-assisted transformations with the initially used ligand either present14 or absent.13,15
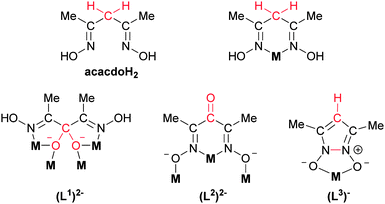
Herein, we present an example of such serendipity in the form of the first example of a polynuclear metal complex, namely the [Cu16(L1)4(L2)8(L3)8]·8H2O (1·8H2O)† wheel, comprising three different forms of acetylacetone dioxime (acacdoH2, Scheme 1), in which the initially employed ligand (acacdoH2) is absent. Indeed, our initial idea was to utilize acacdoH2 for the synthesis of heterometallic 3d–4f clusters acting as either SMMs or Magnetic Refrigerants.
To this end, we reacted CuCl2·2H2O with acacdoH2 in EtOH, to obtain a bright, light green solution. If this solution remains undisturbed, bright green X-ray quality single-crystals of [Cu2Cl4(acacdoH2)2] (2)† are obtained in 75% yield; addition of an aqueous solution of Ln(MeCO2)3·xH2O (Ln = Nd, Gd, Er) into the above solution followed by gentle heating (∼40–50 °C) results in a clear dark green solution. Slow evaporation of the latter affords dark green X-ray quality single-crystals of 1·8H2O in moderate yields (28–37%, depending on the lanthanide ion). Although the lanthanide ion does not appear in the final product, its presence in the reaction mixture is essential since reactions in its absence do not lead to complex 1. Addition of H2O or aqueous solutions of MeCO2M·xH2O (M+ = NH4+, Li+, Na+ or K+) or M′(MeCO2)2·xH2O (M′ = ZnII, CuII, NiII, CoII or MnII) instead of Ln(MeCO2)3·xH2O does not lead to complex 1.
Complex 2 crystallizes in the monoclinic space group C2/c. It comprises a Cu(μ2-Cl)2Cu core with a Cu⋯Cu separation of 3.608 Å (Fig. 1). The two halves of the dimer are related by a crystallographic two-fold axis. The Cu(μ2-Cl)2Cu unit is essentially planar with the Cu and Cl ions deviating from the least-squares plane by 0.083 Å. The geometry around the Cu ions is best described as distorted square pyramidal (τ = 0.17). The basal plane contains two cis N atoms from the acacdoH2 ligand and two cis Cl ions, with the apical site occupied by the Cl2 ion from the other monomer unit. Two intramolecular hydrogen bonds between the oximic OH groups and the terminal Cl1 ions stabilize the dimer. The dimers are further hydrogen bonded through the second oximic OH and the terminal Cl1 ion to form a 1D H-bonded chain along the crystallographic c axis. A salient feature of this structure is that both terminal Cl ions are on the same side of the Cu(μ2-Cl)2Cu plane. Indeed, this is the second example of a molecule in which two terminal Cl ions in a [LClCu(μ2-Cl)2CuClL] (L = N,N-chelate ligand) dimer reside on the same side of the [Cu2] plane.16
Complex 1 crystallizes in the tetragonal space group P4/nnc. The asymmetric unit comprises two Cu ions, one (L3)−, one (L2)2− and half a (L1)2− ligand (Fig. 2 and Scheme 1). The two Cu ions (Cu1 and Cu2) are bridged by one alkoxide (RO−) and an oximato (N–O−) group with a Cu⋯Cu separation of 3.253 Å. Two such dimers are related by a crystallographic two-fold axis passing through C8 of ligand (L1)2− to form a tetranuclear assembly with formula [Cu4(L1)(L2)2(L3)2]. Ligand (L1)2− which is the hydrate of the oxidized form of acacdoH2 bridges all Cu ions within the tetranuclear assembly through the deprotonated hydroxyl groups adopting the μ4-η1:η2:η2:η1 coordination mode; the oximic OH groups remain protonated and are hydrogen bonded to the neighbouring deprotonated oximate O2 atoms of (L2)2− which is the oxidized form of acacdoH2. The latter, (L2)2−, chelates Cu1 through the two oximate N1 and N2 atoms and bridges Cu2 through the deprotonated oximate O1 atom adopting the μ2-η1:η1:η1 coordination mode. The monoanion (L3)− simply chelates Cu2. The second deprotonated oximate O2 atom of (L2)2− is weakly bound to a Cu1 from a neighbouring tetranuclear assembly [Cu1–O2 (1.5 − x, y, 0.5 − z) = 2.548 Å] to form the hexadecanuclear wheel [Cu4(L1)(L2)2(L3)2]4. The overall coordination mode of (L2)2− is thus μ3-η1:η1:η1:η1. The geometry around Cu1 is best described as distorted square pyramidal (τ = 0.32). The basal plane contains two cis N atoms from the (L2)2−, the oximic N3 atom and the O5 atom from the hydrate (L1)2−, with the apical site occupied by the oximate O2 (1.5 − x, y, 0.5 − z) atom of (L2)2− from a neighbouring [Cu4]. Cu2 is in a distorted square planar coordination environment, being chelated by two cis O atoms from (L3)−, the oximate O1 atom and the O5 atom from the hydrate (L1)2−. In the lattice, the molecules of 1 pack in off-set rows along the a and b axes having their [Cu16] mean-planes parallel along the c axis with separations of 8.245 Å and 16.490 Å as shown in Fig. 3. Complex 1 joins a small family of eleven [CuII16] clusters,17,18 three of which are cyclic (wheels or wheel-like).18
 | ||
| Fig. 2 The asymmetric unit of 1 (top), the [Cu4] assembly showing the connections to neighbouring [Cu4] units (middle) and the [Cu16] wheel (bottom). Symmetry codes: (′) 0.5 − y, 0.5 − x, 0.5 − z; (′′) 1.5 − x, y, 0.5 − z; (′′′) 0.5 − y, −1 + x, z. Colour code: same as in Fig. 1. | ||
Although the transformation of acacdoH2 to (L1)2−, (L2)2− and (L3)− was not anticipated, the formation of these anions can be fully rationalized. Methylene moieties (–CH2–) attached to electron withdrawing groups can be aerially oxidized to the corresponding ketones with or without the presence of metal ions.19 In our case the oxidized form of acacdoH2, (L2)2−, is probably metal-assisted since the 1H-NMR spectra of pure acacdoH2 in D2O or CD3OD remains unchanged for several months. Ketones, like (L2)2− may undergo nucleophilic addition of H2O to the carbonyl C atom to form the respective hydrate, (L1)2−. The electrophilic character of the carbonyl C atom may be increased by coordination of the carbonyl O atom to a metal ion (direct polarization) or by coordination of the oximic N or O atoms (induced polarization). Such metal-assisted transformations occur often in py2CO chemistry.12,13 Alternatively, acacdoH2 may first oxidize to the hydrate (H2L1) which upon dehydration forms the ketone form H2L2. Pyrazole N-oxides, like (L3)−, have been previously reported to form by metal-assisted transformations of β-diketone dioximes.20
Dc magnetic susceptibility data for 1 were recorded between 300 and 5 K in an applied field of 1.0 kG. The plots of χMT and χMversus T for 1 are shown in Fig. 4. The χMT value at 300 K is 2.87 cm3 K mol−1 and is significantly lower than the expected spin-only (g = 2) value for 16 non-interacting CuII centres of 6 cm3 K mol−1, suggesting the presence of dominant and strong antiferromagnetic exchange. The χMT product decreases rapidly upon cooling to a value of ∼0.44 cm3 K mol−1 at 100 K and then decreases smoothly until 5 K (0.17 cm3 K mol−1). The low-temperature data denote the presence of ∼2.8% paramagnetic impurity per Cu ion. Considering the structural parameters a 2-J model (inset in Fig. 4) was utilised to fit the experimental data which considers the [Cu16] wheel as four weakly interacting [Cu4] moieties (the magnetic dx2−y2 orbitals of Cu1 and Cu1′′ belonging to neighbouring [Cu4] units being approximately parallel to each other).21 In this model, J1 denotes the exchange pathway between the CuII ions (Cu1⋯Cu2) bridged by one alkoxide (RO−) and an oximato (N–O−) group, and J2 the exchange pathways between the CuII ions (Cu1⋯Cu1′, Cu1⋯Cu2′, Cu2⋯Cu1′ and Cu2⋯Cu2′) bridged by the (CO2) moiety of the hydrate (L1)2−. The experimental data were satisfactorily fitted using the program PHI22 employing the spin Hamiltonian in eqn (1). The best fit (solid lines in Fig. 4) gave the following parameters: J1 = −241.88 cm−1, J2 = −3.42 cm−1, g = 2.18, zJ = −0.027 cm−1, and an impurity = 0.1 (i.e. 2.5% per Cu atom) (zJ describes the intermolecular interactions in a mean field approximation). For such a [Cu4] model this results in a spin ground state S = 0, with the first excited state (S = 1) located ∼483 cm−1 above the ground state. The large difference in the magnitude of J1 and J2 is expected: the former describes a one alkoxo, one oximato bridge which is known to provide very effective superexchange,23 while the latter is a three atom exchange pathway (Cu–O–C–O–Cu) mediated by the hydrate moiety known to mediate weak exchange.24
| Ĥex = −2J1(Ŝ1·Ŝ2 + Ŝ3·Ŝ4) − 2J2(Ŝ1·Ŝ3 + Ŝ1·Ŝ4 + Ŝ2·Ŝ3 + Ŝ2·Ŝ4) | (1) |
 | ||
| Fig. 4 χ M T (○) and χM (□) vs. T plots for complex 1. The solid lines represent the best fits of the experimental data – see text for details. | ||
Our initial forays into the use of acetylacetone dioxime (acacdoH2) as a ligand for the synthesis of polynuclear metal complexes has afforded a dinuclear CuII complex and an aesthetically pleasing hexadecanuclear CuII wheel. The acacdoH2 ligand has been transformed into three different species, all of which are found within the wheel. To the best of our knowledge, the [Cu16] is the first polynuclear metal complex comprising three different forms of a ligand that has undergone metal-assisted transformation, without the originally employed ligand being present in the reaction product. It is becoming apparent that, given the rich reactivity of acacdoH2,20 this ligand may play a major role in the synthesis of a variety of novel polynuclear metal complexes in the future. Indeed, when comparing the coordination chemistry of acacdoH2 with that of py2CO, which has afforded numerous metal-assisted transformations in approximately 40 years of research,12,13 it is self-evident that the former has the potential to surpass the cluster-forming ability of the latter.
Notes and references
- R. E. P. Winpenny, J. Chem. Soc., Dalton Trans., 2002, 1 RSC; V. A. Milway, F. Tuna, A. R. Farrell, L. E. Sharp, S. Parsons and M. Murrie, Angew. Chem., Int. Ed., 2013, 52, 1949 CrossRef CAS PubMed and references cited therein.
- A. J. Tasiopoulos, A. Vinslava, W. Wernsdorfer, K. A. Abboud and G. Christou, Angew. Chem., Int. Ed., 2004, 43, 2117 CrossRef CAS PubMed.
- M. Manoli, R. Inglis, M. J. Manos, V. Nastopoulos, W. Wernsdorfer, E. K. Brechin and A. J. Tasiopoulos, Angew. Chem., Int. Ed., 2011, 50, 4441 CrossRef CAS PubMed.
- G. W. Powell, H. N. Lancashire, E. K. Brechin, D. Collison, S. L. Heath, T. Mallah and W. Wernsdorfer, Angew. Chem., Int. Ed., 2004, 43, 5772 CrossRef CAS PubMed.
- X. J. Kong, Y. Wu, L. S. Long, L. S. Zheng and Z. Zheng, J. Am. Chem. Soc., 2009, 131, 6918 CrossRef CAS PubMed.
- W. G. Wang, A. J. Zhou, W. X. Zhang, M. L. Tong, X. M. Chen, M. Nakano, C. C. Beedle and D. N. Hendrickson, J. Am. Chem. Soc., 2007, 129, 1014 CrossRef CAS PubMed.
- T. Liu, Y. J. Zhang, Z. M. Wang and S. Gao, J. Am. Chem. Soc., 2008, 130, 10500 CrossRef CAS PubMed.
- Z. M. Zhang, S. Yao, Y. G. Li, R. Clerac, Y. Lu, Z. M. Su and E. B. Wang, J. Am. Chem. Soc., 2009, 131, 14600 CrossRef CAS PubMed.
- A. Müller, E. Krickemeyer, S. K. Das, P. Kögerler, S. Sarkar, H. Bögge, M. Schmidtmann and S. Sarkar, Angew. Chem., Int. Ed., 2000, 39, 1612 CrossRef.
- E. E. Moushi, C. Lampropoulos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, J. Am. Chem. Soc., 2010, 132, 16146 CrossRef CAS PubMed.
- W. Wernsdorfer and R. Sessoli, Science, 1999, 284, 133 CrossRef CAS; R. Sessoli and D. Gatteschi, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef PubMed; J. Liu and S. Hill, Polyhedron, 2013, 66, 147 CrossRef PubMed; G. Aromi and E. K. Brechin, Struct. Bonding, 2006, 122, 1 CrossRef; M. Affronte, S. Carretta, G. A. Timco and R. E. Winpenny, Chem. Commun., 2007, 1789 RSC; S. Hill, R. S. Edwards, N. Aliaga-Alcalde and G. Christou, Science, 2003, 302, 1015–1018 CrossRef PubMed; C. J. Milios, R. Inglis, A. Vinslava, R. Bagai, W. Wernsdorfer, S. Parsons, S. P. Perlepes, G. Christou and E. K. Brechin, J. Am. Chem. Soc., 2007, 129, 12505 CrossRef PubMed; C. J. Milios and R. E. Winpenny, Struct. Bonding, 2014 DOI:10.1007/430_2014_149; J.-D. Leng, J.-L. Liu and M.-L. Tong, Chem. Commun., 2012, 48, 5286 RSC; Z.-M. Zhang, L.-Y. Pan, W.-Q. Lin, J.-D. Leng, F.-S. Guo, Y.-C. Chen, J.-L. Liu and M.-L. Tong, Chem. Commun., 2013, 49, 8081 RSC; J.-L. Liu, W.-Q. Lin, Y.-C. Chen, S. Gómez-Coca, D. Aravena, E. Ruiz, J.-D. Leng and M.-L. Tong, Chem. – Eur. J., 2013, 19, 17567 CrossRef PubMed.
- G. S. Papaefstathiou and S. P. Perlepes, Comments Inorg. Chem., 2002, 23, 249 CrossRef CAS; A. J. Tasiopoulos and S. P. Perlepes, Dalton Trans., 2008, 5537 RSC.
- A. D. Katsenis, V. G. Kessler and G. S. Papaefstathiou, Dalton Trans., 2011, 40, 4590 RSC; G. S. Papaefstathiou, A. Escuer, C. P. Raptopoulou, A. Terzis, S. P. Perlepes and R. Vicente, Eur. J. Inorg. Chem., 2001, 1567 CrossRef CAS; A. Dimitrakopoulou, V. Psycharis, C. P. Raptopoulou, A. Terzis, V. Tangoulis and D. P. Kessissoglou, Inorg. Chem., 2008, 47, 7608 CrossRef PubMed.
- K. N. Lazarou, A. K. Boudalis, V. Psycharis and C. P. Raptopoulou, Inorg. Chim. Acta, 2011, 370, 50 CrossRef CAS PubMed.
- M. U. Anwar, Y. Lan, L. M. Beltran, R. Clerac, S. Pfirrmann, C. E. Anson and A. K. Powell, Inorg. Chem., 2009, 48, 5177 CrossRef CAS PubMed; Y. F. Zeng, X. Hu, L. Xue, S. J. Liu, T. L. Hu and X. H. Bu, Inorg. Chem., 2012, 51, 9571 CrossRef PubMed; J. L. Liu, Y. C. Chen, Q. W. Li, S. Gomez-Coca, D. Aravena, E. Ruiz, W. Q. Lin, J. D. Leng and M. L. Tong, Chem. Commun., 2013, 49, 6549 RSC.
- O. González Q, A. M. Atria, E. Spodine, J. Manzur and M. T. Garland, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1993, 49, 1589 CrossRef.
- T. Sugiura, H. Yoshikawa and K. Awaga, Inorg. Chem., 2006, 45, 7584 CrossRef CAS PubMed; L. N. Dawe and L. K. Thompson, Angew. Chem., Int. Ed., 2007, 46, 7440 CrossRef PubMed; V. Chandrasekhar and L. Nagarajan, Dalton Trans., 2009, 6712 RSC; L. N. Dawe, K. V. Shuvaev and L. K. Thompson, Inorg. Chem., 2009, 48, 3323 CrossRef PubMed; T. F. Liu, T. C. Stamatatos, K. A. Abboud and G. Christou, Dalton Trans., 2010, 39, 3554 RSC; N. V. Zauzolkova, M. E. Nikiforova, A. A. Sidorov, I. A. Apolonskaya, M. V. Fedin, V. V. Minin, A. V. Rotov, E. A. Ugolkova, M. A. Kiskin, G. G. Aleksandrov, V. M. Novotortsev and I. L. Eremenko, Izv. Akad. Nauk SSSR, Ser. Khim., 2010, 1161 Search PubMed; A. Adhikary, S. Goswami, J. A. Sheikh and S. Konar, Eur. J. Inorg. Chem., 2014, 963 CrossRef.
- P. Klufers and J. Schuhmacher, Angew. Chem., Int. Ed., 1995, 34, 2119 CrossRef CAS; Y. L. Bai, V. Tangoulis, R. B. Huang, L. S. Zheng and J. Tao, Chem. – Eur. J., 2009, 15, 2377 CrossRef PubMed; G. A. Craig, M. Schutze, D. Aguila, O. Roubeau, J. Ribas-Arino, S. Vela, S. J. Teat and G. Aromi, Inorg. Chem., 2014, 53, 3290 CrossRef PubMed.
- H.-C. Yao, M.-M. Li, G.-S. Yang, Z.-J. Li and Y. Zhu, Inorg. Chim. Acta, 2007, 360, 3959 CrossRef CAS PubMed; F. d. S. Miranda, F. G. Menezes, J. Vicente, A. J. Bortoluzzi, C. Zucco, A. Neves and N. S. Gonçalves, J. Mol. Struct., 2009, 938, 1 CrossRef PubMed; J. Zhang, Z. Zhang, Z. Chen and X. Zhou, Dalton Trans., 2012, 41, 357 RSC.
- A. Kotali and V. P. Papageorgiou, Org. Prep. Proced. Int., 1991, 23, 593 CrossRef CAS.
- R. Ruiz, F. Lloret, M. Julve, J. Faus, M. C. Munoz and X. Solans, Inorg. Chim. Acta, 1998, 268, 263 CrossRef CAS.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164 CrossRef CAS PubMed.
- F. Birkelbach, M. Winter, U. Floerke, H.-J. Haupt, C. Butzlaff, M. Lengen, E. Bill, A. X. Trautwein, K. Wieghardt and P. Chaudhuri, Inorg. Chem., 1994, 33, 3990 CrossRef CAS; A. Escuer, M. S. El Fallah, R. Vicente, N. Sanz, M. Font-Bardia, X. Solans and F. A. Mautner, Dalton Trans., 2004, 1867 RSC; A. S. R. Chesman, D. R. Turner, B. Moubaraki, K. S. Murray, G. B. Deacon and S. R. Batten, Eur. J. Inorg. Chem., 2010, 59 CrossRef.
- A. Chakraborty, B. K. Ghosh, J. Ribas-Arino, J. Ribas and T. K. Maji, Inorg. Chem., 2012, 51, 6440 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: CIF files of complexes 1 and 2, experimental data and tables. CCDC 1026037 and 1026038. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc07582j |
| This journal is © The Royal Society of Chemistry 2014 |