Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Morphology-driven absorption and emission colour changes in liquid-crystalline, cyclometallated platinum(II) complexes†
Valery N.
Kozhevnikov
 *a,
Bertrand
Donnio
bc,
Benoît
Heinrich
b and
Duncan W.
Bruce
*d
*a,
Bertrand
Donnio
bc,
Benoît
Heinrich
b and
Duncan W.
Bruce
*d
aDepartment of Applied Sciences, Northumbria University, Newcastle upon Tyne, NE1 8ST, UK. E-mail: valery.kozhevnikov@northumbria.ac.uk
bInstitut de Physique et Chimie des Matériaux de Strasbourg (IPCMS), UMR 7504 (CNRS-Université de Strasbourg), 23 Rue du Loess BP 43, 67034 Strasbourg Cedex 2, France
cComplex Assemblies of Soft Matter Laboratory (COMPASS), UMI 3254 (CNRS-Solvay-University of Pennsylvania), CRTB, 350 George Patterson Boulevard, Bristol, PA 19007, USA
dDepartment of Chemistry, University of York, Heslington, York YO10 5DD, UK. E-mail: duncan.bruce@york.ac.uk
First published on 24th September 2014
Abstract
Platinum(II) complexes of 1,3-bis(2-pyridyl)benzene containing two alkyl chains are unexpectedly mesomorphic and capable of changing absorption and emission colour depending on the phase obtained after thermal treatment.
Optical properties of square-planar PtII complexes are often determined by the organisation of individual molecules in the bulk.1,2 While in dilute solution absorption and emission are normally determined by individual molecules, in the solid state the way complexes interact can play a crucial role. Molecular aggregates with strong Pt⋯Pt interactions often show drastically different absorption and emission characteristics from other aggregates in which the metal–metal interaction is weak or absent. Such behaviour is explained by the fact that a short Pt–Pt distance allows the formation of metal–metal-to-ligand-charge-transfer (MMLCT) states which leads to a significant red-shift in absorption and emission. Optical responses of this kind have attracted considerable recent interest for the design of sensors and stimulus-responsive materials. Many such materials are based on crystalline samples, but ‘soft’ systems such as gels,3 fibres4 and polymers5 have also been identified. Liquid-crystals are also interesting in this regard. Molecules in the liquid-crystalline state display much higher degrees of (anisotropic) order than classical liquids, but the system is much less ordered than the crystalline state. In addition, the occasional presence of monotropic phases means that kinetically stabilised states can be obtained, sometimes by fast cooling, allowing access to a wider range of self-organised structures. Furthermore, these metastable states can, on occasion, be rather long-lived.6 Liquid-crystalline, cyclometallated complexes of PtII
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7–10 are often highly luminescent and allow observation of morphology-dependent effects in both absorption and emission modes.11 Here we report simple liquid-crystalline PtII complexes of 1,3-bis(2-pyridyl)benzene with just two aliphatic chains, whose thermal behaviour is accompanied by drastic changes both in absorption and emission colour.
7–10 are often highly luminescent and allow observation of morphology-dependent effects in both absorption and emission modes.11 Here we report simple liquid-crystalline PtII complexes of 1,3-bis(2-pyridyl)benzene with just two aliphatic chains, whose thermal behaviour is accompanied by drastic changes both in absorption and emission colour.
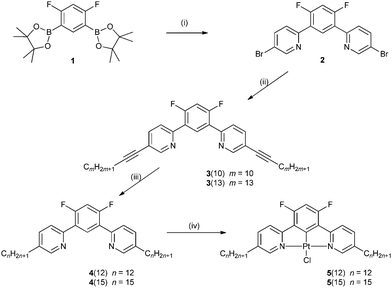
The synthesis of the ligand and the PtII complex are depicted in Fig. 1. A Suzuki–Miyaura coupling of the diboronic acid ester derivative 1 with 2-iodo-5-bromopyridine gives the dibromo derivative 2. This was then reacted with terminal alkynes under Sonogashira conditions to give the alkynyl derivatives, 3, which were then reduced to the alkyl derivatives 4. The target platinum complexes 5, were finally obtained by heating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the ligand and potassium tetrachloroplatinate(II) in acetic acid under reflux.
1 mixture of the ligand and potassium tetrachloroplatinate(II) in acetic acid under reflux.
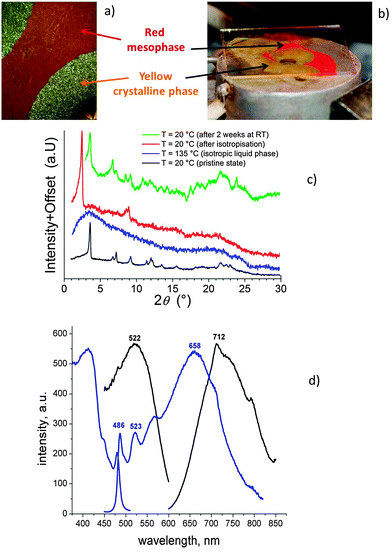
Complexes 5 show interesting thermochromic behaviour. Thus, the yellow solid 5(12), obtained from solution, melts at 119 °C to form a yellow, isotropic liquid. Cooling from the isotropic phase leads to the formation of a viscous, birefringent, monotropic mesophase at 82 °C (Fig. 2a). Interestingly, the formation of the mesophase is accompanied by the change in colour of the sample from yellow to red. The mesophase is fluid immediately after formation but on further cooling progressively becomes more viscous, forming a glassy state at approximately 75 °C without visible changes in appearance. This film stayed red for a period of several days, trapped in the metastable, glassy mesophase. Crystallisation did, however, occur slowly, reforming the crystalline phase and as can be seen in Fig. 2b, this process of reforming the crystalline phase is accompanied by the change in colour from red to yellow. Crystallisation was found to be much faster at temperatures between 75–80 °C, no doubt on account of the greater mobility in the molten phase as the molecules explored different spatial organisation to lead to the crystalline state.
Re-heating the red mesophase then leads to clearing at 93 °C, which is the true thermodynamic transition temperature, and is accompanied by a colour change from red back to yellow. Evidently there is significant supercooling of this transition when the temperature is lowered from the clearing point.
In order to gain a better understanding of the nature of the red and yellow phases, small-angle X-ray scattering (SAXS) experiments were carried out (Fig. 2c). Characteristic of the original yellow crystalline solid obtained from solution showed many sharp reflections with a primary d-spacing = 24.55 Å. While crystallisation prevented collection of data in the fluid red mesophase, a diffraction pattern was obtained in the glassy state and showed a d-spacing = 35.4 Å and a broad reflection close to 2θ = 20°, expected where molten terminal chains are present. There was also evidence for other, broad reflections as well as a sharper reflection corresponding to a d-spacing of about 10 Å. The data are insufficient to assign the phase symmetry, but the combination of the sharper, low-angle reflection and the broad wide-angle reflection supports assignment as a liquid-crystalline phase. This is consistent with the presence of a birefringent texture seen using polarised microscopy (Fig. 2a), although it was not possible to obtain a well-developed texture that would allow mesophase characterisation. If the sample was left to crystallise at room temperature for two weeks, SAXS showed a pattern close to that of the pristine yellow solid with a primary d-spacing = 24.55 Å. The presence of additional reflections suggests the possible co-existence of a second crystalline modification, which may be metastable, being formed during crystallisation.
Similar behaviour was found for complex 5(15) but at slightly different temperatures. Thus, the yellow crystalline solid melts at 120 °C and on cooling, the red, monotropic mesophase begins to form at 90 °C, forming a glass at approximately 80–70 °C on further cooling. The mesophase-isotropic liquid transition takes place at 102 °C on re-heating the red mesophase. Again, crystallisation prevented collection of data in the fluid mesophase.
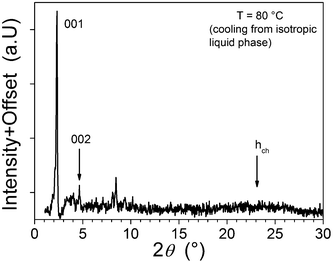
In order to suppress crystallisation, a mixture of 5(12) and 5(15) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by mass) was prepared by dissolving the mixture of compounds in CH2Cl2 followed by evaporation. In this way, crystallisation was suppressed significantly and it was possible to obtain an X-ray pattern for the fluid liquid-crystalline phase (83 °C, Fig. 3). The data can be assigned to a lamellar phase with a periodicity of about 38.5 Å, which distance is reasonable given that the data obtained for pure 5–12 suggested a lamellar phase with d = 35.4 Å. The observation of a mesophase in complexes 5 is perhaps unexpected for such a simple system. This is an interesting phenomena, which deserves further investigation.
1 by mass) was prepared by dissolving the mixture of compounds in CH2Cl2 followed by evaporation. In this way, crystallisation was suppressed significantly and it was possible to obtain an X-ray pattern for the fluid liquid-crystalline phase (83 °C, Fig. 3). The data can be assigned to a lamellar phase with a periodicity of about 38.5 Å, which distance is reasonable given that the data obtained for pure 5–12 suggested a lamellar phase with d = 35.4 Å. The observation of a mesophase in complexes 5 is perhaps unexpected for such a simple system. This is an interesting phenomena, which deserves further investigation.
In term of photophysical properties, both absorption and emission characteristics are affected by the state of the sample (Fig. 2d). The absorption maximum for the yellow crystalline phase is at 410 nm, while for the red phase it is at 520 nm. The luminescence profile also changes from an orange-red colour for the yellow phase (λmax ≈ 486, 658 nm) to the near infrared region (λmax ≈ 730 nm) for the red phase. These data correlate well with previous findings for similar PtII complexes of 1,3-bis(2-pyridyl)benzene that the emission of the yellow phase is the combination of monomer and excimer-like emissions, while the absorption and emission of the red phase is dominated by MMLCT excited state.12
The above data are consistent with the following behaviour. Cooling an isotropic melt of 5 leads to a red, liquid-crystalline phase, the colour of which is indicative of the presence of short Pt⋯Pt interactions judging by characteristic MMLCT absorption and emission.
It has been argued that such metallophilic interactions are responsible for the induction of mesomorphism in related PtII materials.8 Whether these interactions actually drive mesophase formation or whether it is the case that the optimum intermolecular aggregation imposed by mesophase formation leads to a geometry that favours the Pt⋯Pt interactions remains a moot point. However, whatever the mechanism, it is clear that metallophilic Pt⋯Pt interactions are key to the thermochromic behaviour of these complexes. Because thermodynamically the red mesophase is metastable, it eventually reverts to the yellow crystalline phase in which Pt⋯Pt interactions are disrupted. In this way, it is possible to switch the Pt⋯Pt interactions on and off manipulating the sample only thermally without the need for intervention by solvent or mechanical treatment. This binary switch in morphology is in turn translated to a binary switch in both absorption (red/yellow) and emission (NIR/orange) characteristics of the film.
We thank the EU for funding and Johnson Matthey for generous loans of platinum salts.
Notes and references
- K. M.-C. Wong, M. M.-Y. Chan and V. W.-W. Yam, Adv. Mater., 2014, 26, 5558 CrossRef CAS PubMed; J. R. Kumpfer, S. D. Taylor, W. B. Connick and S. J. Rowan, J. Mater. Chem., 2012, 22, 14196 RSC; P. Du, J. Schneider, W. W. Brennessel and R. Eisenberg, Inorg. Chem., 2007, 47, 69 CrossRef PubMed; X.-P. Zhang, T. Wu, J. Liu, J.-X. Zhang, C.-H. Li and X.-Z. You, J. Mater. Chem. C, 2014, 2, 184 RSC; J. Ni, X. Zhang, Y. H. Wu, L. Y. Zhang and Z. N. Chen, Chem. – Eur. J., 2011, 17, 1171 CrossRef PubMed; M. L. Muro, C. A. Daws and F. N. Castellano, Chem. Commun., 2008, 6134 RSC.
- B. Donnio and D. W. Bruce, Liquid crystalline ortho-palladated complexes, in Palladacycles – Synthesis, Characterization and Applications, ed. M. Pfeffer and J. Dupont, Wiley-VCH, Weinheim, 2008 Search PubMed.
- A.-Y. Tam, K.-C. Wong, N. Zhu, G. Wang and V. W.-W. Yam, Langmuir, 2009, 25, 8685 CrossRef CAS PubMed; F. Camerel, R. Ziessel, B. Donnio, C. Bourgogne, D. Guillon, M. Schmutz, C. Iacovita and J.-P. Bucher, Angew. Chem., Int. Ed., 2007, 46, 2659 CrossRef PubMed.
- W. Lu, V. A. L. Roy and C.-M. Che, Chem. Commun., 2006, 3972 RSC; M. Mauro, A. Aliprandi, C. Cebrian, D. Wang, C. Kubel and L. De Cola, Chem. Commun., 2014, 50, 7269 RSC.
- C.-C. Kwok, S.-C. Yu, I. H. T. Sham and C.-M. Che, Chem. Commun., 2004, 2512 RSC.
- H. J. Coles and M. N. Pivnenko, Nature, 2005, 436, 997 CrossRef CAS PubMed.
- S. W. Thomas III, S. Yagi and T. M. Swager, J. Mater. Chem., 2005, 15, 2829 RSC; K. Venkatesan, P. H. J. Kouwer, S. Yagi, P. Mueller and T. M. Swager, J. Mater. Chem., 2008, 18, 400 RSC; A. Santoro, A. C. Whitwood, J. A. G. Williams, V. N. Kozhevnikov and D. W. Bruce, Chem. Mater., 2009, 21, 3871 CrossRef CAS; Y. Wang, Y. Liu, J. Luo, H. Qi, X. Li, M. Nin, M. Liu, D. Shi, W. Zhu and Y. Cao, Dalton Trans., 2011, 40, 5046 RSC; M. Spencer, A. Santoro, G. R. Freeman, A. Diez, P. R. Murray, J. Torroba, A. C. Whitwood, L. J. Yellowlees, J. A. G. Williams and D. W. Bruce, Dalton Trans., 2012, 41, 14244 RSC.
- T. Hegmann, J. Kain, S. Diele, B. Schubert, H. Bögel and C. Tschierske, J. Mater. Chem., 2003, 13, 991 RSC; C. Damm, G. Israel, T. Hegmann and C. Tschierske, J. Mater. Chem., 2006, 16, 1808 RSC; Y. Wang, Y. Liu, J. Luo, H. Qi, X. Li, M. Nin, M. Liu, D. Hu and Y. Cao, Dalton Trans., 2011, 40, 5046 RSC.
- M. Krikorian, S. Liu and T. M. Swager, J. Am. Chem. Soc., 2014, 136, 2952 CrossRef CAS PubMed.
- C. T. Liao, H. H. Chen, H. F. Hsu, A. Poloek, H. H. Yeh, Y. Chi, K. W. Wang, C. H. Lai, G. H. Lee, C. W. Shih and P. T. Chou, Chem. – Eur. J., 2011, 17, 546 CrossRef CAS PubMed; Y. Li, A. Y.-Y. Tam, K. Wong, M.-C. W. Li, L. Wu and V. W.-W. Yam, Chem. – Eur. J., 2011, 17, 8048 CrossRef PubMed; H.-C. Chang, T. Shiozaki, A. Kamata, K. Kishida, T. Ohmori, D. Kiriya, T. Yamauchi, H. Furukawa and S. Kitagawa, J. Mater. Chem., 2007, 17, 4136 RSC.
- V. N. Kozhevnikov, B. Donnio and D. W. Bruce, Angew. Chem., Int. Ed., 2008, 47, 6286 CrossRef CAS PubMed.
- E. Rossi, A. Colombo, C. Dragonetti, D. Roberto, F. Demartin, M. Cocchi, P. Brulatti, V. Fattori and J. A. G. Williams, Chem. Commun., 2012, 48, 3182 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, DSC and SAXS data. See DOI: 10.1039/c4cc06958g |
| This journal is © The Royal Society of Chemistry 2014 |