Open Access Article
Open Access ArticleDirected one-pot syntheses of crown ether wheel-containing main chain-type polyrotaxanes with controlled rotaxanation ratios†
Kazuko
Nakazono
,
Tomonori
Ishino
,
Tomoyuki
Takashima
,
Daisaku
Saeki
,
Daisuke
Natsui
,
Nobuhiro
Kihara
and
Toshikazu
Takata
*
Department of Organic and Polymeric Materials, Tokyo Institute of Technology, 2-12-1, O-okayama, Meguro-ku, Tokyo 152-8552, Japan. E-mail: ttakata@polymer.titech.ac.jp
First published on 13th October 2014
Abstract
The directed synthesis of main chain-type polyrotaxanes possessing crown ether wheels was successfully achieved through two methods, A and B. Method A involved the direct wheel threading of poly(sec-ammonium salt) followed by end-capping with a bulky group, while method B utilized polyaddition of a pseudo[2]rotaxane monomer to facilitate the control of the structure, i.e. the rotaxanation ratio.
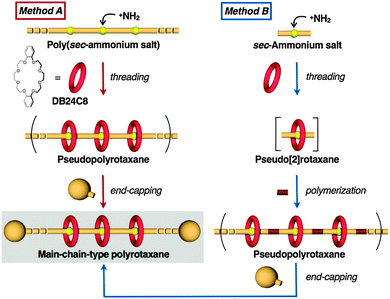
The first synthesis of a polypseudorotaxane (PPRX) from cyclodextrin (CD) and a linear polymer by Ogata1 and its end-capping to give a polyrotaxane (CD-PRX) by Harada2 heralded significant progress in the science of main chain-type CD-PRX, opening the door to various applications3 such as cross-linked gels.4 Efficient syntheses of CD-PRX have now led to its actual use.5 However, little is known about PRXs having other wheel components,6,7 in particular crown ether (CE) wheels, despite Gibson's pioneering work on PRXs.8 Therefore, the synthesis of PRX (not poly[2]rotaxane9) with a sufficiently high or controlled CE content and effective end-cap groups is an important challenge in this area.8a,10 CE–ammonium-salt-type rotaxane chemistry has enabled the synthesis of end-capped [5]-6d and [20]rotaxanes;6a,b however, both rotaxanes exhibit low solubility because of their oligoionic structures. Since CE–ammonium interaction-driven self-assembly often achieves accurate supramolecular systems, the greater ease with which functional groups can be introduced into CE as compared to CD, as has been proven in rotaxane chemistry,11 strongly indicates the potential utility of CE-based main chain-type PRX (CE-PRX), and hence, its synthesis becomes of paramount importance. We have long studied the development of an effective synthetic method for CE-PRX with a controlled structure, and have found effective synthetic methods.12 A recent Grubbs' simple approach to CE-PRX via the polymerization of a CE-based pseudo[2]rotaxane monomer,13 has prompted us to report our results on the successful synthesis of CE-PRX with controlled structures. We report herein the directed one-pot synthesis of CE-PRXs by two reliable synthetic protocols to yield structure-definite PRXs with controlled number of wheel components and effective end-cap groups (Fig. 1), building on our recent results on the synthesis of dibenzo-24-crown-8-ether (DB24C8)-containing PRXs.14 We also describe the simple modification to nonionic PRX that enables size-exclusion-chromatographic (SEC) measurements.
Fig. 1 shows the two strategies for the synthesis of CE-PRXs. Method A consists of the initial wheel threading of poly(sec-ammonium salt) and subsequent end-cap with a bulky agent. In the method B, copolymerization of pseudo[2]rotaxane monomer and appropriate comonomer to CE-PRX by the successive end-cap to facilitate the control of the structure, i.e. the rotaxanation ratio.
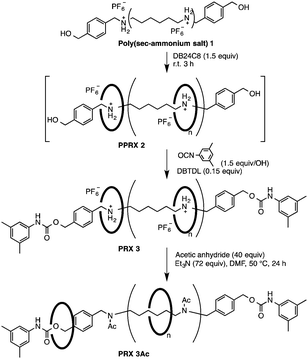
Method A: direct wheel threading of poly(sec-ammonium salt) followed by end-capping. We started with this synthetic protocol because rotaxane synthesis by this route is usually known to proceed efficiently in one-pot,15 and it seems to be suitable for giving high-molecular-weight PRX. Although poly(sec-ammonium salt) and the corresponding CE-PRX seemed insoluble in ordinary organic solvents because of their polyionic structures, we expected to observe CE-PRX formation because the oligorotaxane is more soluble than its axle polymer precursor oligo(sec-ammonium salt).6a,b We chose poly(hexamethyleneammonium PF6) (PHMAP) as the axle polymer because the hexamethylene spacer distance between the ammonium groups is appropriate for a high-yield synthesis of [3]rotaxane.6e PHMAP was obtained by the reduction of 6,6-nylon with BH3–THF complex16 followed by treatment with NH4PF6. PHMAP was relatively insoluble in organic solvents and the corresponding PPRX was obtained in only a minimal yield with DB24C8 in a CHCl3–CH3CN mixed solvent system. To address this problem, benzyl ammonium salt moieties were introduced at both PHMAP termini to enhance complexation capability.15,17 4-Hydroxymethylbenzyl-terminated PHMAP 1 was prepared by condensation of amine-terminated 6,6-nylon and methyl 4-formylbenzoate followed by reduction and subsequent treatment with NH4PF6. The molecular weight of 1 was calculated by 1H NMR to be Mn = 3200. One-pot synthesis of PRX 3via intermediate PPRX 2 was carried out as follows (Scheme 1): a heterogeneous mixture of 1 and DB24C8 was allowed to stand and then 3,5-dimethylphenylisocyanate and a catalytic amount of dibutyltindilaurate (DBTDL) in solvent at room temperature was added to it15 (Table 1). The structure of PRX 3 was confirmed by spectroscopic analyses.
The reactions proceeded under heterogeneous conditions even with polar solvents such as CH3CN and CH3NO2 (Table 1). For PRX 3, the yield increased to 76% with increasing solvent polarity and hence the solubility of PPRX 2 during the end-capping process. For 1, the rotaxanation ratio (RR) of the NH2 group was sufficiently high (>92%) without solvent dependency. These results suggest the following important reaction features: (i) the formation of PPRX 2 is accelerated by introducing a more strongly interactive unit (benzyl group) with CE at the axle termini;18 (ii) complexation between 1 and CE proceeds under heterogeneous conditions; and (iii) the CE wheel can enter into the center of the axle polymer chain. For PRX 3, neutralization by N-acetylation,19 which is required for estimating Mn, was incomplete, presumably because CE is densely packed because of its short CE–CE distance.6c To address this issue, we turned to method B.
Method B: step polymerization of a reactive pseudo[2]rotaxane monomer followed by end-capping. We designed this synthetic protocol because of the reported efficiency of end-capping pseudo[2]rotaxane to give [2]rotaxane15 in addition to the high solubility of the monomer.6e,20
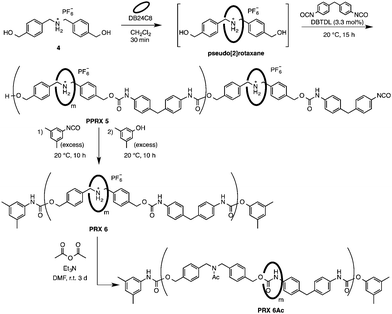
We chose a dibenzylammonium-type axle 417 because of the enhanced stability of the corresponding pseudorotaxane monomer. One-pot synthesis of PRX 6 was carried out as follows (Scheme 2): a mixture of pseudo[2]rotaxane (derived in situ from 4 and DB24C8) and diphenylmethane diisocyanate was treated with DBTDL in CH2Cl2. The resulting PPRX 5 was treated successively with 3,5-dimethylphenylisocyanate and 3,5-dimethylphenol to give PRX 6 selectively in high yield. The RR value and Mn were determined by 1H NMR (Table 2).
| Entry | DB24C8/NH2 (equiv.) | Yieldb (%) | RRc (%) | PRX 6 (NMR) | PRX 6Ac (SEC) | ||
|---|---|---|---|---|---|---|---|
| M n/104/Da | M n/104 /Da | M w/104/Da | PDI | ||||
| a 4 = 0.5 M. b Calculated on the basis of 4. c Determined by 1H NMR (range of error ±2%). d Estimated by SEC (DMF, PSt) after N-acetylation. | |||||||
| 1 | 2.0 | 93 | 95 | 2.6 | 2.8 | 6.0 | 3.6 |
| 2 | 1.0 | 84 | 90 | 2.5 | 4.2 | 9.3 | 2.2 |
| 3 | 0.5 | 84 | 55 | 1.4 | 3.3 | 7.4 | 2.2 |
Inspection of Table 2 reveals that method B is versatile and gives the desired CE-based PRXs in high yield. The RR values were controllable by the DB24C8 feed ratio; for example, 0.5–2.0 equiv. of DB24C8 yielded PRX 6 with RR values in the range 55–95%. The end-capped structure of PRX 6 was confirmed by a decomposition study of PRX 6 in DMSO-d6, which indicated that no DB24C8 was liberated from PRX 6 at ambient temperature.21 The structure of PRX 6 was determined using NMR, IR, and SEC. The 1H NMR spectra clearly indicate the involvement of the rotaxane structure, with DB24C8 localized at the ammonium moieties (Fig. 2). All signals are reasonably assignable; the two signals around 4.6 (A) and 4.8 (a) ppm indicate uncomplexed and complexed N-benzyl protons, respectively, whose ratio corresponds to the RR value.
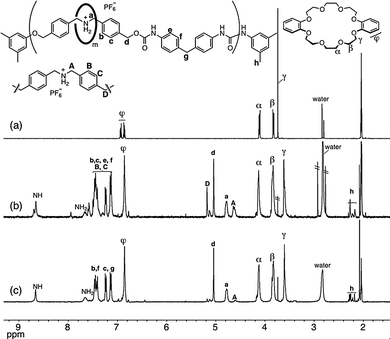 | ||
| Fig. 2 Partial 1H NMR spectra of (a) DB24C8, (b) PRX 6 (RR = 55%), and (c) PRX 6 (RR = 95%) (400 MHz, (CD3)2CO). | ||
To remove the polyionic nature from PRX 6, we subjected PRX 6 to N-acetylation with acetic anhydride and triethylamine.19 The acetylation reaction proceeded very efficiently to yield PRX 6Ac (93% yield, Table 2, entry 1). Table 2 shows the SEC results of PRX 6Acs with different rotaxanation ratios (RR). All PRX 6Acs gave unimodal SEC profiles to support the occurrence of complete N-acetylation of the ammonium moieties of PRX 6s, agreeing with other spectral data.22 The 1H NMR spectrum of PRX 6Acs suggested two sets of signals assignable to the protons around the urethane moiety, although the axle component is symmetric. Thus, the conversion of the ammonium moiety caused the positional change of the crown ether wheel from the ammonium moiety to the urethane moiety probably due to the hydrogen bonding interaction as same as the model [2]rotaxane S9.22 This is the first synthesis of a nonionic DB24C8-based main chain-type polyrotaxane. The solubility of the PRXs in typical organic solvents was investigated. PRX 3 was soluble in some polar solvents such as DMF and CH3CN, whereas PRX 6 and PRX 6Ac showed the higher solubility to various solvents including acetone, as we expected.22 In addition, the high molecular weight of PRX 6 was confirmed by its good film-forming property, which resulted in the formation of flexible transparent films by casting from the acetone solutions (Fig. 3).22
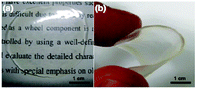 | ||
| Fig. 3 Photographs of a transparent thin film of PRX 6 (RR = 95%) prepared by casting from the acetone solution. | ||
In conclusion, we have successfully accomplished the directed one-pot synthesis of a challenging target polymer in the PRX family: CE-based main chain-type PRXs. We showed that CE-PRXs are unusually soluble in typical organic solvents such as DMF, despite their polyionic structures. Method B, involving step polymerization of pseudo[2]rotaxane monomers, enables the controlled synthesis of PRXs possessing the desired rotaxanation ratios (RR values). Complete neutralization by the N-acetylation of the ammonium moieties that promotes the CE translation on the axle23 proceeded remarkably well. A variety of modifications of CEs established so far enable versatile functionalization of PRXs. Thus, the present study heralds continuing rapid progress in the synthesis and application of CE-PRXs.
This work was financially supported by a Grant-in-Aid for Scientific Research from MEXT, Japan (No. 18064008 and 23245031), and K. N. thanks the Global COE program (Education and Research Center for Material Innovation) for the support.
Notes and references
- N. Ogata, K. Sanui and J. Wada, J. Polym. Sci., Polym. Lett. Ed., 1976, 14, 459 CrossRef CAS.
- A. Harada and M. Kamachi, Macromolecules, 1990, 23, 2821 CrossRef CAS.
- (a) J. Li and X. J. Loh, Adv. Drug Delivery Rev., 2008, 60, 1000 CrossRef CAS PubMed; (b) S. Loethen, J.-M. Kim and D. H. Thompson, Polym. Rev., 2007, 47, 383 CrossRef CAS; (c) T. Ooya and N. Yui, MML Ser., 2006, 7, 231 CAS; (d) M. J. Frampton and H. L. Anderson, Angew. Chem., Int. Ed., 2007, 46, 1028 CrossRef CAS PubMed; (e) A. Harada, A. Hashidzume, H. Yamaguchi and Y. Takashima, Chem. Rev., 2009, 109, 5974 CrossRef CAS PubMed.
- (a) J. Araki and K. Ito, Soft Matter, 2007, 3, 1456 RSC; (b) Y. Okumura and K. Ito, Adv. Mater., 2001, 13, 485 CrossRef CAS.
- (a) H. Iguchi, S. Uchida, Y. Koyama and T. Takata, ACS Macro Lett., 2013, 2, 527 CrossRef CAS; (b) K. Jang, K. Miura, Y. Koyama and T. Takata, Org. Lett., 2012, 14, 3088 CrossRef CAS PubMed; (c) K. Nakazono, T. Takashima, T. Arai, Y. Koyama and T. Takata, Macromolecules, 2010, 43, 691 CrossRef CAS; (d) T. Arai, M. Hayashi, N. Takagi and T. Takata, Macromolecules, 2009, 42, 1881 CrossRef CAS; (e) N. Kihara, K. Hinoue and T. Takata, Macromolecules, 2005, 38, 223 CrossRef CAS; (f) J. Araki, C. Zhao and K. Ito, Macromolecules, 2005, 38, 7524 CrossRef CAS; (g) S. Loethen, T. Ooya, H. S. Choi, N. Yui and D. H. Thompson, Biomacromolecules, 2006, 7, 2501 CrossRef CAS PubMed; (h) K. Kato, H. Komatsu and K. Ito, Macromolecules, 2010, 43, 8799 CrossRef CAS.
- (a) M. E. Belowich, C. Valente, R. A. Smaldone, D. C. Friedman, J. Thiel, L. Cronin and J. F. Stoddart, J. Am. Chem. Soc., 2012, 134, 5243 CrossRef CAS PubMed; (b) J. Wu, K. C.-F. Leung and J. F. Stoddart, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 17266 CrossRef CAS PubMed; A. L. Fuller, D. A. Leigh and P. J. Lusby, Angew. Chem., Int. Ed., 2007, 46, 5015 Search PubMed; (c) K. M. Wollyung, K. Xu, M. Cochran, A. M. Kasko, W. L. Mattice, C. Wesdemiotis and C. Pugh, Macromolecules, 2005, 38, 2574 CrossRef CAS; (d) N. Watanabe, T. Yagi, N. Kihara and T. Takata, Chem. Commun., 2002, 2720 RSC; (e) T.-A. Yamagishi, A. Kawahara, J. Kita, M. Hoshima, A. Umehara and S. Ishida, Macromolecules, 2001, 34, 6565 CrossRef CAS; (f) D. Whang, Y.-M. Jeon, J. Heo and K. Kim, J. Am. Chem. Soc., 1996, 118, 11333 CrossRef CAS.
- Polypseudorotaxane (without end-cap group): (a) P. L. Vidal, M. Billon, B. Divisia-Blohorn, G. Bidan, B. Divisia-Blohorn, J. M. Kern and J. P. Sauvage, Chem. Commun., 1998, 629 RSC; (b) S. S. Zhu and T. M. Swager, J. Am. Chem. Soc., 1997, 119, 12568 CrossRef CAS; (c) G. J. Owen and P. Hodge, Chem. Commun., 1997, 11 RSC; (d) P. E. Mason, I. W. Parsons and M. S. Tolley, Angew. Chem., Int. Ed., 1996, 35, 2238 CrossRef CAS.
- (a) F. Huang and H. W. Gibson, Prog. Polym. Sci., 2005, 30, 982 CrossRef CAS; (b) F. M. Raymo and J. F. Stoddart, Chem. Rev., 1999, 99, 1643 CrossRef CAS PubMed; (c) H. W. Gibson, S. Liu, P. Lecavalier, C. Wu and Y. X. Shen, J. Am. Chem. Soc., 1995, 117, 852 CrossRef CAS; (d) C. Wu, M. C. Bheda, C. Lim, Y. X. Shen, J. Sze and H. W. Gibson, Polym. Commun., 1991, 32, 204 CAS.
- Poly[2]rotaxane synthesis: (a) T. Ikeda, M. Higuchi and D. G. Kurth, J. Am. Chem. Soc., 2009, 131, 9158 CrossRef CAS PubMed; (b) D. Tuncel and J. H. G. Steinke, Macromolecules, 2004, 37, 288 CrossRef CAS; (c) T. J. Kidd, T. J. A. loontjens, D. A. Leigh and J. K. Y. Wong, Angew. Chem., Int. Ed., 2003, 42, 3379 CrossRef CAS PubMed.
- Higher order rotaxanated CE-PPRX (without end-caps): (a) D. Loveday, G. L. Wilkes, M. C. Bheda, Y. X. Shen and H. W. Gibson, J. Macromol. Sci., Part A: Pure Appl. Chem., 1995, 32, 1 CrossRef; (b) H. W. Gibson, C. Wu, Y. X. Shen, M. Bheda, J. Sze, P. Engen, A. Prasad, H. Marand, D. Loveday and G. Wilkes, Polym. Prepr., 1991, 32, 637–638 CAS. (with end-caps): (c) S.-H. Chiu, S. J. Rowan, S. J. Cantrill, L. Ridvan, P. R. Ashton, R. L. Garrell and J. F. Stoddart, Tetrahedron, 2002, 58, 807 CrossRef CAS.
- (a) H. Sasabe and T. Takata, J. Porphyrins Phthalocyanines, 2007, 11, 334 CrossRef CAS; (b) Y.-G. Lee, Y. Koyama, M. Yonekawa and T. Takata, Macromolecules, 2010, 43, 4070 CrossRef CAS.
- T. Ishino, K. Nakazono, Y. Koyama and T. Takata, Polym. Prepr., 2009, 58, 2876 Search PubMed.
- N. Momčilovič, P. G. Clark, A. J. Boydston and R. H. Grubbs, J. Am. Chem. Soc., 2011, 133, 19087 CrossRef PubMed.
- Y. Abe, H. Okamura, S. Uchida and T. Takata, Polym. J., 2014, 46, 553 CrossRef CAS.
- Y. Furusho, H. Sasabe, D. Natsui, K. Murakawa, T. Takata and T. Harada, Bull. Chem. Soc. Jpn., 2004, 77, 179 CrossRef CAS.
- T. Perner and R. C. Schultz, Br. Polym. J., 1987, 19, 181 CrossRef CAS.
- K a [Bn2NH2·PF6-DB24C8] = 420 M−1, Ka [n-Bu2NH2·PF6-DB24C8] = 50 M−1 (298 K, CD3CN): P. R. Ashton, P. J. Campbell, E. J. T. Chrystal, P. T. Glink, S. Menzer, D. Philp, N. Spencer, J. F. Stoddart, P. A. Tasker and D. J. Williams, Angew. Chem., 1995, 107, 1997 ( Angew. Chem., Int. Ed. , 1995 , 34 , 1865 ) CrossRef.
- The CE is perhaps likely to translate to the inner ammonium site on the axle rather than dethreading to gain in enthalpy.
- (a) Y. Tachibana, H. Kawasaki, N. Kihara and T. Takata, J. Org. Chem., 2006, 71, 5093 CrossRef CAS PubMed; (b) N. Kihara, Y. Tachibana, H. Kawasaki and T. Takata, Chem. Lett., 2000, 506 CrossRef CAS.
- H. Kawasaki, N. Kihara and T. Takata, Chem. Lett., 1999, 1015 CrossRef CAS.
- Measurable dissociation of polypseudorotaxne was confirmed in DMSO at room temperature.
- See ESI†.
- Wheel components move in each phase between acetyl–acetyl groups that are not sequential on the axle polymer: N. Kihara, Y. Koike and T. Takata, Chem. Lett., 2007, 36, 208 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectra, SEC profiles, and DSC profiles. See DOI: 10.1039/c4cc06943a |
| This journal is © The Royal Society of Chemistry 2014 |