Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
New magnetic frameworks of [(CuF2(H2O)2)x(pyz)]†
A.
Lanza
ab,
C.
Fiolka
a,
M.
Fisch
ab,
N.
Casati
b,
M.
Skoulatos
c,
C.
Rüegg
cd,
K. W.
Krämer
a and
Piero
Macchi
 *a
*a
aDepartment of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, 3012 Bern, Switzerland
bSwiss Light Source, Paul Scherrer Institute, CH-5232 Villigen, Switzerland
cLaboratory for Neutron Scattering and Imaging, Paul Scherrer Institute, CH-5232 Villigen, Switzerland
dDPMC-MaNEP, University of Geneva, CH-1211 Geneva, Switzerland
First published on 29th September 2014
Abstract
Pressure-driven orbital reordering in the quantum magnet [CuF2(H2O)2(pyz)], (pyz = pyrazine), dramatically affects its magnetic exchange interactions. The crystal chemistry of this system is enriched with a new phase above 3 GPa, surprisingly concomitant with other polymorphs. Moreover, we discovered an unprecedented compound with a different stoichiometry, [(CuF2(H2O)2)2(pyz)], featuring magnetic bi-layers.
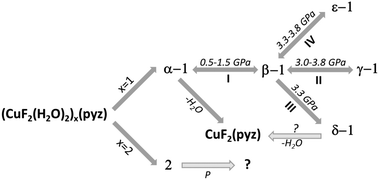
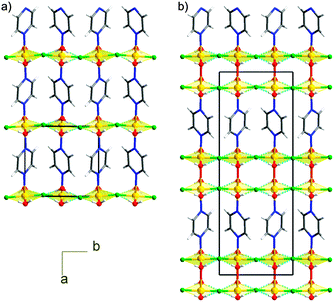
In the past few years, [CuF2(H2O)2(pyz)] (1) has been extensively investigated due to its rapidly evolving phase diagram as a function of pressure and, consequently, an easy control of its magnetism.1–3 In the monoclinic P21/c ambient pressure structure, α-1,4 the three ligands pairwise occupy the trans positions of a distorted octahedron around Cu. By connecting pairs of Cu2+ ions, the pyrazine ligands build a mono-dimensional (1D) coordination polymer along the crystallographic a axis. These chains pack in a tight 2D supramolecular network of O–H⋯F hydrogen bonds expanded in the bc plane (Fig. 1) producing a quasi-2D magnetic network.2 The N–Cu bonds are rather elongated, i.e. they produce a pseudo Jahn–Teller axis.5 This implies that the singly occupied d orbital of Cu (the magnetic orbital) is perpendicular to this direction and thus involves H2O and F− but not pyz (as confirmed by the spin density maps we calculated, Fig. S3 in ESI†). A previous powder X-ray diffraction (P-XRD) study2 has shown that 1 undergoes two successive, pressure-induced, isosymmetric phase transitions of the first order, both of which switch the stretched Cu–X bond direction, implying an orbital reordering. In fact, at around 1 GPa, phase transition I (see Scheme 1) produces β-1, whose octahedron is elongated along the H2O–Cu–OH2 direction. Water molecules no longer interact with the magnetic orbital (Fig. S4, ESI†) and therefore the exchange path is just 1D, propagating along the Cu–pyz–Cu chains. A second phase transition (II, in Scheme 1) was observed at around 3.3 GPa yielding γ-1, for which the Cu–F bonds are now elongated.2 Although the structure of γ-1 has been refined only using a very rigid model and the magnetism has not been investigated, it was sensibly assumed that the magnetic orbital lies in the Cu(pyz)2(H2O)2 plane, hindering the coupling of magnetic centers through the H-bonds and maintaining the same β-1-type 1D network. This sequence of phase transitions replicates the spectrochemical series of the ligands (pyz > H2O > F−).
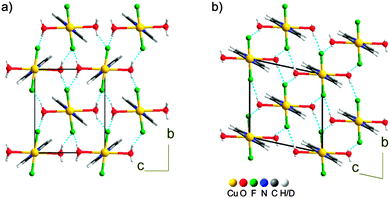 | ||
| Fig. 1 Comparison between the crystal packing of 1 in phases β (a) and ε (b). The hydrogen bond network in the bc plane is shown with blue dashed lines. | ||
On the other hand, in a more recent single crystal X-ray diffraction (SC-XRD) study, Prescimone et al.3 identified a new triclinic phase (δ-1) at 3.3 GPa after a first order phase transition (III, in Scheme 1). δ significantly differs from α, β or γ as it features Cu–F–Cu bridges obtained from the nucleophilic substitution by a fluoride ligand on one water molecule, which leaves the Cu coordination sphere and occupies an extra-framework site. The elongated direction of the Cu octahedron is Fbridge–Cu–H2O, an intermediate step along the spectrochemical series. At variance with I and II, reversibility of transition III has not been reported and is very likely impossible.
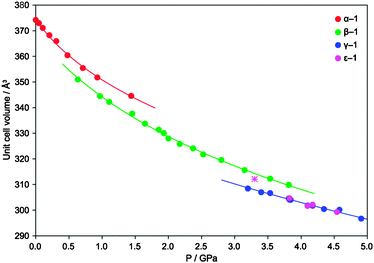
In order to explore the rich solid-state chemistry of 1, we undertook a study aimed at explaining the factors governing the dichotomy at 3.3 GPa. In fact, the powder experiments described in ref. 2 were carried out using isopropanol as the pressure transmitting medium (PTM), whereas for the single crystal experiments described in ref. 3, petrol ether was used. Thus, we performed extensive X-ray diffraction investigations on single crystal and powder samples using different PTMs. For most experiments we used crystals of the fully deuterated compound, but in order to maintain consistency and fluency throughout the manuscript we will not explicitly distinguish H from D, unless necessary. All experiments confirmed the occurrence of phase transition I, in a rather large pressure range at around 1.0 GPa (Fig. 2) with co-existence of β-1 and α-1, depending on the experimental conditions, in particular on the pressure increase rate (i.e. ΔP/Δt, where ΔP is the pressure increase and Δt is the time between one measurement and the next, including equilibration). Upon further compression, we discovered and characterized by SC-XRD a new polymorph, ε-1, obtained from β-1 at ca. 3.3 GPa, which is the same pressure previously reported for the occurrence of both γ-1 and δ-1. This transformation (IV, in Scheme 1) is also a first order phase transition, reproducible under several conditions.
In ε-1, the intra- and inter-molecular connectivity of β-1 is retained, although the symmetry is lowered to triclinic (Table 1).6 The asymmetric unit comprises one copper ion, located at an inversion center, one water molecule, one fluoride and half pyz, building a distorted octahedron. The distinctive feature of ε-1 is the reorientation of the pyz rings, which are all parallel to each other within the chain and among neighboring chains, whereas, in all other known phases of 1, neighboring chains alternate two orientations. The compression also causes the pyz planes to deviate from the Cu⋯Cu direction, forming an angle of approximately 10° compared to ca. 4° in β-1, (Fig. S1 in ESI†). The pseudo Jahn-Teller axis is unvaried from β-1, the Cu–O bond remaining the longest (Cu–O 2.360(12), Cu–N 2.01(2), Cu–F 1.885(5) Å). Therefore, phase transition IV is not the consequence of an orbital reordering. This is confirmed by periodic density functional calculations7 that indicate spin density accumulation on Cu (0.75), on each F (0.05) and on each pyrazine (0.12 overall), see Fig. S6 in the ESI.† No transformation of ε-1 was observed, at least up to 4.4 GPa, indicating that the H2O–Cu–OH2 direction remains the elongated one up to this pressure. The hydrogen bond pattern is similar to those of phases α–γ (Fig. 1) and the H2O⋯F distances are comparable to those of β-1. Therefore, the magnetic exchange interactions must be 1D, in analogy with β-1.2
| Phase name | α-1 | β-1 | γ-1 | δ-1 | ε-1 | 2 |
|---|---|---|---|---|---|---|
| a Z is intended as the number of the corresponding [(CuF2(H2O)2)x(pyz)] formula units per unit cell. | ||||||
| Crystal system | Monoclinic | Triclinic | Triclinic | Monoclinic | ||
| Space group, Za | P21/c, 2 |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , 3 , 3 |
A![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , 2 , 2 |
I2/c, 4 | ||
| Technique | P-XRD | SC-XRD | SC-XRD | SC-XRD | ||
| P (GPa) | 0.0001 | 1.1 | 3.8 | 3.3 | 3.3 | 0.0001 |
| Unit cell dimensions: | ||||||
| a (Å) | 7.6893(3) | 6.8703(4) | 6.8112(6) | 6.7987(8) | 6.813(4) | 21.0200(3) |
| b (Å) | 7.5655(1) | 7.6758(1) | 7.6632(6) | 10.452(6) | 7.1823(11) | 7.55300(10) |
| c (Å) | 6.9001(3) | 7.1103(5) | 6.5422(8) | 7.272(2) | 7.4463(12) | 6.88100(10) |
| α (°) | 90 | 90 | 90 | 86.24(3) | 84.344(15) | 90 |
| β (°) | 111.205(5) | 114.108(7) | 117.318(10) | 115.844(16) | 116.72(4) | 98.456(2) |
| γ (°) | 90 | 90 | 90 | 88.71(2) | 78.85(3) | 90 |
| V (Å3) | 374.22(3) | 342.25(4) | 303.39(6) | 463.3(3) | 312.1(2) | 1080.58(3) |
| V mol (cm3 mol−1) | 112.70 | 103.07 | 91.37 | 93.02 | 93.99 | 162.71 |
| Reference | This study | This study | This study | 3 | This study | This study |
Complementary P-XRD measurements confirmed the occurrence of phase transition IV above 3.2 GPa, though always concomitant with transition II, making phases β, γ and ε co-existing up to 3.8 GPa. None of these experiments gave any unambiguous evidence of δ-1, so far obtained only from the single crystal experiments by Prescimone et al.3 The simultaneous occurrence of three phase changes (II, III and IV) is thermodynamically forbidden, therefore kinetic effects must play an important role during these transformations. In fact, we found that larger pressure increase rates favor γ-1, which was the only product when the pressure was directly increased to 3.8 GPa. The purity of this sample enabled us to refine its structure from P-XRD data using a more flexible model (see the ESI† for details). The elongation of Cu–F proposed by Halder et al.2 was confirmed (2.215(16) Å), in agreement with periodic DFT calculations (2.198 Å) and the spin density maps prove the re-orientation of the magnetic orbital (see Fig. S5, ESI†), which does not involve F atoms.
The surprising behavior of 1 is complemented by the discovery of [(CuF2(H2O)2)2(pyz)] (2, Fig. 3), obtained for the first time as a second crystalline fraction from several solutions under ambient conditions. 2 was also found to grow epitaxially on large crystals of 1 stored in their mother liquor. The crystal structure of 2 is monoclinic I2/c (ref. 6) and comprises one moiety of [CuF2(H2O)2(pyz)0.5] in the asymmetric unit. One pyz, two F− and three H2O molecules coordinate to the Cu2+ centers in a distorted octahedral fashion. The pseudo Jahn–Teller axis is N–Cu–Obridge suggesting that the magnetic orbital is located in the CuF2O2 plane as in α-1 (Cu–O 2.5210(13), Cu–N 2.4041(15), Cu–O′ 1.9922(13), Cu–O′′ 1.9653(14), Cu–F 1.8987(10), and Cu–F′ 1.8936(10) Å). Two H2O molecules act as bridges in the equatorial-axial position between the Cu ions, thus building pairs of edge-sharing distorted octahedra (Fig. S5, ESI†). Overall, a 1D coordination polymer, extended along the crystallographic a axis, is formed in which each pyz links two Cu2F4(H2O)4 dimeric moieties (Fig. 3). The N–Cu–OH2 bond is slightly tilted with respect to the translational direction of the polymer. The aromatic rings assume alternate orientations along the same chain. As for 1, the chains of 2 are packed in a 2D supramolecular network of H-bonds in the bc plane which involves all the water and the fluoride ligands (Fig. S7c, ESI†) with H2O⋯F distances comparable to those of α-1. Table 1 summarizes relevant crystallographic information.
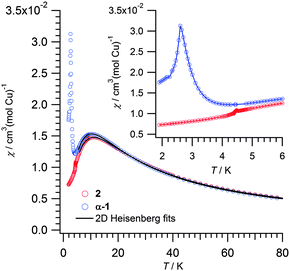
The magnetic susceptibility χ of 2 (in deuterated form) shows a broad maximum at 11 K, due to short range antiferromagnetic correlations. At lower T, χ declines and features a faint peak at 4.5 K. In comparison, χ of α-1 has a broad maximum at 10.5 K and a sharp peak at 2.6 K (Fig. 4). At 250 K, χT is 0.478 and 0.458 emu K mol−1 for 2 and α-1, respectively, both higher than the spin-only moment of Cu2+ (g = 2; 0.375 emu K mol−1). For both 2 and α-1, χT declines with decreasing T, slightly until 60 K and more significantly thereafter (Fig. S8, ESI†). Curie–Weiss fits result in negative Weiss θ values, confirming the dominant antiferromagnetic interactions (Table 2).
Both compounds exhibit 2D Cu2+ layers, hence it is adequate to characterize them as spin 1/2 Heisenberg square lattices, with the Hamiltonian H = −Jij∑Si·Sj. The results of the fitting for these quantum Heisenberg antiferromagnets are shown in Table 2. Fitted J values are consistent with the mean-field expression |2kBTmax/JS(S + 1)| = 2.53,8 that would predict |J| = 11.6 K and 11.1 K for 2 and α-1, respectively, as well as with the literature values reported for α-1.1b Since the 2D network is similar, it is reasonable to find comparable exchange parameters 2 and α-1. Further proof of the robustness of this H-bonded network is given by CuF2(H2O)2(3-chloropyridine).8 As shown in Fig. 5, the peak at 4.5 K in the magnetic susceptibility of 2 is gradually suppressed by the increasing field, which is typical for spin-canting,8 whereas the broad maximum at 11 K is field-independent. The steep decline of χ below the peak can be understood as an antiferromagnetic order between the bi-layers. In α-1 long range order (LRO) is observed at 2.6 K.1b The higher LRO temperature of 2 can be regarded as a consequence of the bi-layer present in 2 and distinct from α-1. Indeed bridging water molecules occupying equatorial-axial positions have been found to cause small magnetic coupling, but were seldom studied before.9
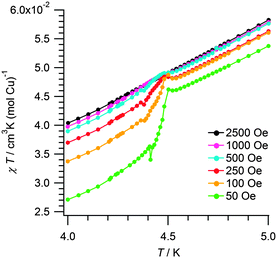 | ||
| Fig. 5 Magnetic field dependence of the ordering temperature in 2. χT vs. T measured at different fields. | ||
This study demonstrates that the [(CuF2(H2O)2)x(pyz)] system has an even richer phase diagram than reported so far. A new high-pressure polymorph, ε-1, was discovered and structurally characterized and the structure of γ-1 was better elucidated. Interestingly, 1 is now known to form three different phases at the same pressure (ca. 3.3 GPa). Such abundance of easily accessible polymorphs implies very similar free energies and important kinetic effects. Preliminary experiments using different pressure increase rates and equilibration times, indicate that ε-1 forms only when compressed under quasi-reversible conditions, whereas γ-1 is the only product if the compression is very rapid.10 On the other hand, the fact that δ-1 was not reproduced by us under any experimental conditions suggests that the more radical transformation III might not be stimulated only by pressure. Depending on the conditions, [CuF2(H2O)2(pyz)] rapidly gives crystalline [CuF2(pyz)],11 thus δ-1 could be regarded as an intermediate of a dehydration reaction, in which one of the water molecules, replaced by F−, leaves the Cu coordination sphere but remains trapped in the crystal. Noteworthily, the experiment by Prescimone et al.3 was carried out on a single crystal, where the dehydration might have occurred more slowly especially if the sample was kept under pressure.
Another outcome of our study is the discovery of compound 2, which is complementary to δ-1 and CuF2(H2O)2,12 having water instead of fluorine bridges. 2 differs from 1 in the stoichiometry, and the main structural difference is the alternation of pyz and double (instead of single) Cu(H2O)2F2 layers linked by bridging water molecules. 2 is a 2D Heisenberg antiferromagnet and the bi-layer feature results in a higher LRO temperature.
Further investigations are currently in progress in order to analyze the role of kinetics, radiation exposure and sample preparation in triggering the alternative phase transitions and reactions. Moreover, the high-pressure behavior of 2 and its magnetic structure are currently being investigated.
We thank Dr. Vincent Olieric for the help in performing the P-XRD experiments at X06DA in a non-conventional setup and Urs Kämpfer for the elemental analysis. The Swiss National Science Foundation supported this research under projects 144534, 132877, and 150257.
Notes and references
- (a) M. Conner, A. McConnell, J. Schlueter and J. Manson, J. Low Temp. Phys., 2006, 142, 273 CrossRef CAS; (b) J. L. Manson, M. M. Conner, J. A. Schlueter, A. C. McConnell, H. I. Southerland, I. Malfant, T. Lancaster, S. J. Blundell, M. L. Brooks, F. L. Pratt, J. Singleton, R. D. McDonald, C. Lee and M.-H. Whangbo, Chem. Mater., 2008, 20, 7408 CrossRef CAS; (c) J. A. Schlueter, H. Park, J. L. Manson, H. Nakotte and A. J. Schultz, Phys. B, 2010, 405, S324 CrossRef CAS PubMed; (d) J. L. Musfeldt, Z. Liu, S. Li, J. Kang, C. Lee, P. Jena, J. L. Manson, J. A. Schlueter, G. L. Carr and M. H. Whangbo, Inorg. Chem., 2011, 50, 6347 CrossRef CAS PubMed; (e) C. H. Wang, M. D. Lumsden, R. S. Fishman, G. Ehlers, T. Hong, W. Tian, H. Cao, A. Podlesnyak, C. Dunmars, J. A. Schlueter, J. L. Manson and A. D. Christianson, Phys. Rev. B, 2012, 86, 064439 CrossRef; (f) S. Ghannadzadeh, J. S. Moeller, P. A. Goddard, T. Lancaster, F. Xiao, S. J. Blundell, A. Maisuradze, R. Khasanov, J. L. Manson, S. W. Tozer, D. Graf and J. A. Schlueter, Phys. Rev. B, 2013, 87, 241102 CrossRef.
- G. J. Halder, K. W. Chapman, J. A. Schlueter and J. L. Manson, Angew. Chem., Int. Ed., 2011, 50, 419 CrossRef CAS PubMed.
- A. Prescimone, C. Morien, D. Allan, J. A. Schlueter, S. W. Tozer, J. L. Manson, S. Parsons, E. K. Brechin and S. Hill, Angew. Chem., Int. Ed., 2012, 51, 7490 CrossRef CAS PubMed.
- We propose a homogeneous labelling of the phases of 1, based on the use of greek letters, as is common use for identifying polymorphs.
- L. R. Falvello, J. Chem. Soc., Dalton Trans., 1997, 4463 RSC.
- The non-conventional crystallographic setting was chosen in order to allow straightforward comparison of the new phase with the other crystal structures.
- R. Dovesi, V. R. Saunders, C. Roetti, R. Orlando, C. M. Zicovich-Wilson, F. Pascale, B. Civalleri, K. Doll, N. M. Harrison, I. J. Bush, P. D'Arco and M. Llunell, CRYSTAL09 User's Manual, University of Torino, Torino, 2009 Search PubMed.
- S. H. Lapidus, J. L. Manson, H. Park, A. J. Clement, S. Ghannadzadeh, P. Goddard, T. Lancaster, J. S. Möller, S. J. Blundell, M. T. F. Telling, J. Kang, M.-H. Whangbo and J. A. Schlueter, Chem. Commun., 2013, 49, 499 RSC.
- D. Ghoshal, T. K. Maji, G. Mostafa, S. Sain, T. H. Lu, J. Ribas, E. Zangrando and N. R. Chaudhuri, Dalton Trans., 2004, 1687 RSC.
- M. Fisch, A. Lanza, N. Casati and P. Macchi, in preparation.
- S. H. Lapidus, J. L. Manson, J. Liu, M. J. Smith, P. Goddard, J. Bendix, C. V. Topping, J. Singleton, C. Dunmars, J. F. Mitchella and J. A. Schlueter, Chem. Commun., 2013, 49, 3558 RSC.
- (a) S. C. Abrahams and E. Prince, J. Chem. Phys., 1962, 36, 50 CrossRef CAS PubMed; (b) S. C. Abrahams, J. Chem. Phys., 1962, 36, 56 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental section and results. CCDC 998110 and 998111. For the ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc06696k |
| This journal is © The Royal Society of Chemistry 2014 |