Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
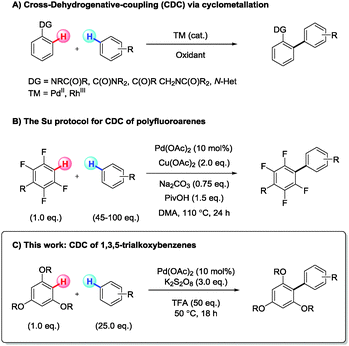
Palladium catalysed cross-dehydrogenative-coupling of 1,3,5-trialkoxybenzenes with simple arenes†
Thomas E.
Storr
,
Faridah
Namata
and
Michael F.
Greaney
*
School of Chemistry, University of Manchester, Oxford Rd, Manchester M13 9PL, UK. E-mail: michaelgreaney@manchester.ac.uk
First published on 8th September 2014
Abstract
Pd-catalysed cross-dehydrogenative coupling of 1,3,5-trialkoxybenzenes with simple aromatic hydrocarbons is reported. The method enables the coupling of two aromatic C–H positions to generate multi-ortho-substituted biaryls.
There is great current interest in the discovery of new C–H cross-coupling reactions with improved atom economy and substrate scope.1 Cross-dehydrogenative coupling (CDC), where C–C bond formation takes place at two C–H sites on different molecules, represents an ideal transformation in this regard. No pre-functionalisation is required on either coupling partner, creating exciting possibilities for rapid and economic synthesis. The oxidative homo-coupling of arenes is well known, with precedent stretching back to the 19th century for stoichiometric metal couplings,2 and with many more recent reports describing transition metal catalysed processes in the presence of cheap oxidants.3 Extending this idea to encompass two distinct C–H coupling partners, however, remains a major challenge.4 Notable advances in this area include Kita's hypervalent iodine mediated couplings,5 the CDC of acidic heteroarenes or polyfluorobenzenes6 with aromatic solvents7 and other heteroarenes,8 and the use of directing groups to effect chelation controlled metallation and subsequent coupling.9 Lu and co-workers have shown that naphthalene10 can be effectively cross-coupled with simple aromatics using PdII catalysis. Extension to other substrates, however, gave poor selectivities and low yields. These reports illustrate the potential power of CDC for arene synthesis, encouraging us to investigate the feasibility of metal-catalysed CDC of two electron rich arenes in the absence of chelating groups, a transformation with little precedent (Scheme 1).
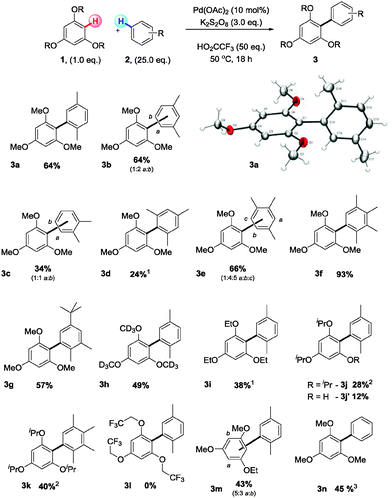
Using the Lu conditions as a starting point, we investigated the CDC of 1,3,5-trimethoxybenzene (1) (limiting reagent) with para-xylene (2a) (solvent and super stoichiometric reagent), to form the penta substituted biaryl 3a (Scheme 2). A comprehensive screen of reaction parameters (see ESI†) established the following reaction conditions (25.0 eq. simple arene, 10 mol% Pd(OAc)2, 3.0 eq. K2S2O8 and 50.0 eq. TFA, at 50 °C for 18 h), producing 3a in 64% yield. Biaryl 3a was characterised by single crystal X-ray crystallography,11 showing the highly congested-tri-ortho-substituted biaryl axis to possess an average torsion angle of 83.8(8)° (Scheme 2). In most reactions performed in this study, the concurrent production of homo-coupled 2 (2,2′,5,5′-tetramethyl-1,1′-biphenyl and 1,4-dimethyl-2-(4-methylbenzyl)benzene) was observed along with 3a, but no homo-coupled or benzylated products of 1 were observed.3l Further investigations revealed that the CDC reaction proceeds at lower temperatures, even down to 0 °C, albeit in lower yields. Reproducibility issues were, however, evident at lower temperatures and a reaction temperature of 50 °C was found to provide consistent and reproducible results.
Following reaction optimisation, an assessment of both arene substrates was performed. The use of para- and meta-xylene both provided synthetically useful quantities of 3a and 3b (64% yield), whereas ortho-xylene was less successful yielding only 34% of the desired biaryl product 3c. Likewise, when moving to more sterically hindered aromatic hydrocarbons significantly reduced yields were observed; with mesitylene as the coupling partner only 24% of 3d could be obtained. These results are not surprising, as the synthesis of tetra-ortho-substituted biaryls is a significant challenge and usually necessitates the use of specialised catalyst–ligand combinations.12 The reaction of pseudocumene (1,2,4-trimethylbenzene) with 1 proceeded smoothly to supply 3e in 66% yield as a mixture of isomers. Interestingly, prehenitene (1,2,3,4-tetramethylbenzene) could be employed to great effect yielding 3f in an excellent yield of 93%. When multiple C–H bonds on the aromatic hydrocarbon solvent are available for arylation multiple isomeric products are observed (3b, 3c and 3e). Use of 4-tert-butyl-ortho-xylene, however, gave the sterically least-hindered biaryl 3g in good yield as a single isomer. Aromatic solvents bearing electron withdrawing groups could not be coupled to 1.
Turning to the alkoxyarene partner, a necessity for the 1,3,5-substitution pattern was noted, with additional substituents not being tolerated, presumably due to the increased steric congestion. Symmetrical 1,3,5-trialkoxybenzenes gave the CDC product in most cases, but increasing the steric bulk on the alkoxymoiety (Me < Et < iPr, 3a, 3i, 3j) led to a steady reduction in reaction yield 64% to 28%. The CDC product of 1,3,5-triisopropoxybenzene and para-xylene (3j) was accompanied by 12% of the ortho-dealkylated product 3j′ (see ESI† for details). This dealkylation is likely to be an acid promoted post-coupling side reaction, given the selectivity and the fact that 3,5-dimethoxyphenol is not a competent substrate. Again, when employing prehenitene as the coupling partner to 1,3,5-triisopropoxybenzene an increased yield of the CDC product, 3k, was obtained in comparison to using para-xylene. The reaction also proved sensitive to the electronic character of the alkoxyarene component, with the trifluoro analogue of 1,3,5-triethoxybenzene failing to react (3l). An unsymmetrical 1,3,5-trialkoxybenene substrate was competent in the CDC reaction, affording 3m in moderate yield as a mixture of isomers. CDC of 1 with benzene was not possible under the established reaction conditions; however, a reduction in the quantity of trifluoroacetic acid in the reaction mixture (5.0 eq.), and using palladium(II) trifluoroacetate as the pre-catalyst proved successful, giving 3n in 45% yield.
Having a successful CDC protocol in hand, a number of control reactions and mechanistic probes were performed in order to gain a greater insight into the reaction mechanism. The CDC reaction of 1a with para-xylene (2a) does not proceed in the absence of the palladium catalyst or TFA. In the absence of the oxidant the reaction only produces trace quantities (<10%, approximately) of the desired product. The intermolecular kinetic isotope effect was determined to be 1.0 using a competition reaction between 2a and d10-2a, which produced 3a and d9-3a in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (see ESI† for further details on the KIE determination experiments). Significant incorporation of hydrogen, derived from TFA, was observed at the 4′- and 6′-positions but not the 3′-position of the dimethylphenyl moiety. This D/H exchange is likely to occur post arylation, supported by the fact that H/D exchange almost exclusively occurs at the ortho and para positions to the electron rich aryl unit. This result is consistent with an electrophilic palladation mechanism whereby the breaking of the C–H bond is not rate limiting and likely happens via loss of a proton from a Wheland type arenium intermediate.13 The KIE of the trimethoxybenzene component could not be ascertained due to facile D/H exchange, indeed, simple stirring of 1 in D2O generates d3-1a.14
1 ratio (see ESI† for further details on the KIE determination experiments). Significant incorporation of hydrogen, derived from TFA, was observed at the 4′- and 6′-positions but not the 3′-position of the dimethylphenyl moiety. This D/H exchange is likely to occur post arylation, supported by the fact that H/D exchange almost exclusively occurs at the ortho and para positions to the electron rich aryl unit. This result is consistent with an electrophilic palladation mechanism whereby the breaking of the C–H bond is not rate limiting and likely happens via loss of a proton from a Wheland type arenium intermediate.13 The KIE of the trimethoxybenzene component could not be ascertained due to facile D/H exchange, indeed, simple stirring of 1 in D2O generates d3-1a.14
With the information gained about the CDC of 1,3,5-trialkoxybenzenes with simple arenes we would like to propose a tentative mechanism for this transformation (Scheme 3). The in situ generated palladium(II) trifluoroacetate (A) can be nucleophilically attacked by the electron rich arene (1). The electrophilic palladation of 1 should be a facile process due to the highly electron rich aromatic ring of 1.
After loss of a proton from the metallo-Wheland intermediate a palladium(II) arene species (B) is generated. B can then be intercepted by another aryl-component in a second, likely slower, palladation step to provide a diaryl palladium(II) species (C). There are now two possibilities to obtain the desired product from intermediate C; (1) reductive elimination to generate the new C–C bond and palladium(0) which can then be rapidly re-oxidised by the peroxydisulfate salt or (2) C could be oxidised by the peroxydisulfate anion up to a transient diaryl palladium(IV) species15 which would swiftly reductively eliminate 3 regenerating the catalytically active species in the process (see ESI†).
It is also feasible that an oxidation of the palladium(II) catalyst up to an intermediate palladium(IV) could occur prior to C–H palladation, these processes have been reported but only in some highly specific examples.16 Although the possibility of radical mediated processes in action within this reaction system cannot be ruled out without further studies, we believe that this is less likely.17
In conclusion, we have developed a new method for the CDC of 1,3,5-trialkoxy benzenes with simple aromatic hydrocarbons, accessing a number of novel highly hindered tri- and tetra-ortho-substituted biaryls in a single step. This is the first account of a high yielding protocol for the C–H/C–H cross-coupling of two disparate electron rich benzenes, and further applications are underway in our laboratory.
We thank the University of Manchester and the EPSRC for funding (Leadership Fellowship to M.F.G.), J. Raftery (University of Manchester) for X-ray crystallographic analysis, and the EPSRC mass spectrometry service at the University of Swansea.
Notes and references
- (a) J.-Q. Yu and Z. Shi, Top. Curr. Chem., 2010, 292 Search PubMed; (b) L. Ackermann, Modern Arylation Methods, Wiley-VCH, Weinheim, 2009 Search PubMed.
- (a) J. Z. Löwe, Z. Chem., 1868, 4, 603 Search PubMed; (b) V. von Richter, Ber. Dtsch. Chem. Ges., 1873, 6, 1249 CrossRef; (c) A. P. Dianin, Zh. Russ. Fiz.-Khim. O-va., 1874, 183 Search PubMed; (d) J. P. Kovacic and M. B. Jones, Chem. Rev., 1987, 87, 357 CrossRef.
- (a) R. van Helden and G. Verberg, Recl. Trav. Chim. Pays-Bas, 1965, 84, 1263 CrossRef CAS; (b) J. M. Davidson and C. Triggs, J. Chem. Soc. A, 1968, 1324 RSC; (c) M. O. Unger and R. A. Fouty, J. Org. Chem., 1969, 34, 18 CrossRef CAS; (d) H. Iataaki and H. Yoshimoto, J. Org. Chem., 1973, 38, 76 CrossRef; (e) F. R. S. Clark, R. O. C. Norman, C. B. Thomas and J. S. Willson, J. Chem. Soc., Perkin Trans. 1, 1974, 1289 RSC; (f) T. Itahara, M. Hashimoto and H. Yumisashi, Synthesis, 1984, 255 CrossRef CAS; (g) M. Okamoto and T. Yamaji, Chem. Lett., 2001, 212 CrossRef CAS; (h) T. Yokota, S. Sakaguchi and Y. Ishii, Adv. Synth. Catal., 2002, 344, 849 CrossRef CAS . For recent relevant examples see: ; (i) Y. Rong, R. Li and W. Lu, Organometallics, 2007, 26, 4376 CrossRef CAS; (j) Y. Izawaa and S. S. Stahl, Adv. Synth. Catal., 2010, 352, 3223 CrossRef PubMed; (k) D. G. Pintori and M. F. Greaney, Org. Lett., 2011, 13, 5713 CrossRef CAS PubMed; (l) L. Zhou and W. Lu, Organometallics, 2012, 31, 2124 CrossRef CAS.
- (a) Y. Wu, J. Wang, F. Mao and F. Y. Kwong, Chem. – Asian J., 2014, 9, 26 CrossRef CAS PubMed; (b) W. Han and A. R. Ofial, Synlett, 2011, 1951 CAS; (c) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS PubMed; (d) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed; (e) C. J. Scheuermann, Chem. – Asian J., 2010, 5, 436 CrossRef CAS PubMed; (f) J. A. Ashenhurst, Chem. Soc. Rev., 2010, 39, 540 RSC; (g) C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed.
- (a) T. Dohi, M. Ito, K. Morimoto, M. Iwata and Y. Kita, Angew. Chem., Int. Ed., 2008, 47, 1301 CrossRef CAS PubMed; (b) Y. Kita, K. Morimoto, M. Ito, C. Ogawa, A. Goto and T. Dohi, J. Am. Chem. Soc., 2009, 131, 1668 CrossRef CAS PubMed; (c) T. Dohi, M. Ito, I. Itani, N. Yamaoka, K. Morimoto, H. Fujioka and Y. Kita, Org. Lett., 2011, 13, 6208 CrossRef CAS PubMed; (d) T. Dohi, T. Kamitanaka, S. Watanabe, Y. Hu, N. Washimi and Y. Kita, Chem. – Eur. J., 2012, 18, 13614 CrossRef CAS PubMed; (e) K. Morimoto, K. Sakamoto, Y. Ohnishi, T. Miyamoto, M. Ito, T. Dohi and Y. Kita, Chem. – Eur. J., 2013, 19, 8726 CrossRef CAS PubMed; (f) M. Ito, H. Kubo, I. Itani, K. Morimoto, T. Dohi and Y. Kita, J. Am. Chem. Soc., 2013, 135, 14078 CrossRef CAS PubMed.
- (a) Y. Wei and W. Su, J. Am. Chem. Soc., 2010, 132, 16377 CrossRef CAS PubMed; (b) H. Li, J. Liu, C.-L. Sun, B.-J. Li and Z.-J. Shi, Org. Lett., 2011, 13, 276 CrossRef CAS PubMed.
- (a) D. R. Stuart, E. Villemure and K. Fagnou, J. Am. Chem. Soc., 2007, 129, 12072 CrossRef CAS PubMed; (b) D. R. Stuart and K. Fagnou, Science, 2007, 316, 1172 CrossRef CAS PubMed; (c) T. A. Dwight, N. R. Rue, D. Charyk, R. Josselyn and B. DeBoef, Org. Lett., 2007, 9, 3137 CrossRef CAS PubMed; (d) S. Potavathri, K. C. Pereira, S. I. Gorelsky, A. Pike, A. P. LeBris and B. DeBoef, J. Am. Chem. Soc., 2010, 132, 14676 CrossRef CAS PubMed; (e) C.-Y. He, S. Fan and X. Zhang, J. Am. Chem. Soc., 2010, 132, 12850 CrossRef CAS PubMed; (f) C. C. Malakar, D. Schmidt, J. Conrad and U. Beifuss, Org. Lett., 2011, 13, 1378 CrossRef CAS PubMed; (g) D. G. Pintori and M. F. Greaney, J. Am. Chem. Soc., 2011, 133, 1209 CrossRef CAS PubMed; (h) A. N. Campbell, E. B. Meyer and S. S. Stahl, Chem. Commun., 2011, 47, 10257 RSC; (i) F. Chen, Z. Feng, C.-Y. He, H.-Y. Wang, Y.-l. Guo and X. Zhang, Org. Lett., 2012, 14, 1176 CrossRef CAS PubMed; (j) G. Wu, J. Zhou, M. Zhang, P. Hu and W. Su, Chem. Commun., 2012, 48, 8964 RSC; (k) Z. Li, L. Ma, J. Xu, L. Kong, X. Wu and H. Yao, Chem. Commun., 2012, 48, 3763 RSC; (l) N. A. B. Juwaini, J. K. P. Ng and J. Seayad, ACS Catal., 2012, 2, 1787 CrossRef CAS; (m) C.-Y. He, Q.-Q. Min and X. Zhang, Organometallics, 2012, 31, 1335 CrossRef CAS.
- (a) P. Xi, F. Yang, S. Qin, D. Zhao, J. Lan, G. Gao, C. Hu and J. You, J. Am. Chem. Soc., 2010, 132, 1822 CrossRef CAS PubMed; (b) X. Gong, G. Song, H. Zhang and X. Li, Org. Lett., 2011, 13, 1766 CrossRef CAS PubMed; (c) W. Han, P. Mayer and A. R. Ofial, Angew. Chem., Int. Ed., 2011, 50, 2178 CrossRef CAS PubMed; (d) Z. Wang, F. Song, Y. Zhao, Y. Huang, L. Yang, D. Zhao, J. Lan and J. You, Chem. – Eur. J., 2012, 18, 16616 CrossRef CAS PubMed; (e) S. Fan, Z. Chen and X. Zhang, Org. Lett., 2012, 14, 4950 CrossRef CAS PubMed; (f) J. Dong, Y. Huang, X. Qin, Y. Cheng, Y. J. Hao, D. Wan, W. Li, X. Liu and J. You, Chem. – Eur. J., 2012, 18, 6158 CrossRef CAS PubMed; (g) C.-Y. He, Z. Wang, C.-Z. Wu, F.-L. Qing and X. Zhang, Chem. Sci., 2013, 4, 3508 RSC; (h) W. Liu, Y. Li, Y. Wang and C. Kuang, Org. Lett., 2013, 15, 4682 CrossRef CAS PubMed; (i) X.-P. Fua, Q.-Q. Xuana, L. Liua, D. Wanga, Y.-J. Chena and C.-J. Li, Tetrahedron, 2013, 69, 4436 CrossRef PubMed; (j) N. Salvanna, G. C. Reddy and B. Das, Tetrahedron, 2013, 69, 2220 CrossRef CAS PubMed; (k) B. Liu, Y. Huang, J. Lan, F. Song and J. You, Chem. Sci., 2013, 4, 2163 RSC; (l) X. Chen, X. Huang, Q. He, Y. Xie and C. Yang, Chem. Commun., 2014, 50, 3996 RSC; (m) Y. Shang, X. Jie, H. Zhao, P. Hu and W. Su, Org. Lett., 2014, 16, 416 CrossRef CAS PubMed.
- (a) K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2007, 129, 11904 CrossRef CAS PubMed; (b) G. Brasche, J. García-Fortanet and S. L. Buchwald, Org. Lett., 2008, 10, 2207 CrossRef CAS PubMed; (c) X. Zhao, C. S. Yeung and V. M. Dong, J. Am. Chem. Soc., 2010, 132, 5837 CrossRef CAS PubMed; (d) C. S. Yeung, X. Zhao, N. Borduas and V. M. Dong, Chem. Sci., 2010, 1, 331 RSC; (e) M. Kitahara, N. Umeda, K. Hirano, T. Satoh and M. Miura, J. Am. Chem. Soc., 2011, 133, 2160 CrossRef CAS PubMed; (f) C. S. Yeung and V. M. Dong, Synlett, 2011, 0974 CAS; (g) X. Wang, D. Leow and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 13864 CrossRef CAS PubMed; (h) J. Wencel-Delord, C. Nimphius, F. W. Patureau and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 2247 CrossRef CAS PubMed; (i) J. Wencel-Delord, C. Nimphius, H. Wang and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 13001 CrossRef CAS PubMed.
- R. Li, L. Jiang and W. Lu, Organometallics, 2006, 25, 5973 CrossRef CAS.
- CCDC 1015999 contains the crystallographic data for 3a. ORTEP-3 was used to produce the thermal ellipsoid plots: L. J. Farrugia, J. Appl. Crystallogr., 1997, 30, 565 CrossRef CAS.
- (a) J. M. Saá and G. Martorell, J. Org. Chem., 1993, 58, 1963 CrossRef; (b) J. Yin, M. P. Rainka, X.-X. Zhang and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 1162 CrossRef CAS PubMed; (c) S. D. Walker, T. E. Barder, J. R. Martinelli and S. L. Buchwald, Angew. Chem., Int. Ed., 2004, 43, 1871 CrossRef CAS PubMed; (d) L. Ackermann, H. K. Potukuchi, A. Althammer, R. Born and P. Mayer, Org. Lett., 2010, 12, 1004 CrossRef CAS PubMed.
- (a) A. D. Ryabov, I. K. Sakodinskaya and A. K. Yatsimirsky, J. Chem. Soc., Dalton Trans., 1985, 2629 RSC; (b) S. I. Gorelsky, Coord. Chem. Rev., 2013, 257, 153 CrossRef CAS PubMed.
- Recent work from Stahl and co-workers identified a bi-metallic mechanism in operation for the oxidative homo-coupling of o-xylene (catalytic Pd(OAc)2, O2, AcOH), which featured extremely large KIEs (>20): D. Wang, Y. Izawa and S. S. Stahl, J. Am. Chem. Soc., 2014, 136, 9914 CrossRef CAS PubMed.
- P. Sehnal, R. J. K. Taylor and I. J. S. Fairlamb, Chem. Rev., 2010, 110, 824 CrossRef CAS PubMed.
- (a) J. M. Racowski, N. D. Ball and M. S. Sanford, J. Am. Chem. Soc., 2011, 133, 18022 CrossRef CAS PubMed; (b) A. Maleckis, J. W. Kampf and M. S. Sanford, J. Am. Chem. Soc., 2013, 135, 6618 CrossRef CAS PubMed.
- For examples of radical involvement in palladium catalysis see: (a) W.-Y. Yu, W. N. Sit, K.-M. Lai, Z. Zhou and A. S. C. Chan, J. Am. Chem. Soc., 2008, 130, 3304 CrossRef CAS PubMed; (b) C.-W. Chan, Z. Zhou, A. S. C. Chan and W.-Y. Yu, Org. Lett., 2010, 12, 3926 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterisation data for all new compounds. CCDC 1015999. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc06271j |
| This journal is © The Royal Society of Chemistry 2014 |