Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Proton reduction by molecular catalysts in water under demanding atmospheres†
David W.
Wakerley
,
Manuela A.
Gross
and
Erwin
Reisner
*
Christian Doppler Laboratory for Sustainable SynGas Chemistry, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, UK. E-mail: reisner@ch.cam.ac.uk
First published on 10th November 2014
Abstract
The electrocatalytic proton reduction activity of a Ni bis(diphosphine) (NiP) and a cobaloxime (CoP) catalyst has been studied in water in the presence of the gaseous inhibitors O2 and CO. CoP shows an appreciable tolerance towards O2, but its activity suffers severely in the presence of CO. In contrast, NiP is strongly inhibited by O2, but produces H2 under high CO concentrations.
The implementation of an artificial photosynthetic system would offer a sustainable route to clean and storable energy.1 This process could generate H2 fuel, or a gas mixture of H2 and CO, known as syngas, which can be used to produce long-chain hydrocarbons or methanol.2 Proton reduction catalysts are an integral part of either system and have consequently generated considerable research interest.3
Inhibitor tolerance under real-world operating conditions is a vital trait for a proton reduction catalyst, but has received relatively little attention to date. Depending on the intended use of a system, proton reduction catalysts could be exposed to large amounts of O2 (through water splitting) or CO (CO2 splitting). Trace amounts of such inhibitors typically poison the most active H2 evolution catalysts, such as platinum4 and H2-producing enzymes (hydrogenases).5,6
Molecular synthetic catalysts offer an alternative route to proton reduction7–9 and it has recently emerged that some molecular catalysts are tolerant towards O2 in aqueous solution. This observation has prompted a number of contemporary studies into H2 evolution under aerobic conditions. Co-based complexes make up the majority of these O2 tolerant species; cobaloximes were the first earth-abundant catalysts shown to be functional under air,10 followed by a Co–corrole catalyst11 and a Co-microperoxidase.12
Recently, a rationally designed bis(1,5-R′-diphospha-3,7-R′′-diazacyclooctane)Ni catalyst from DuBois and co-workers has set a new benchmark for H2 production activity.13 Derivatives of this Ni catalyst have since been able to generate considerable amounts of H2 from aqueous solutions,14,15 an important step in the development of water-splitting systems.16 However, inhibition remains unexplored for this promising type of catalyst.
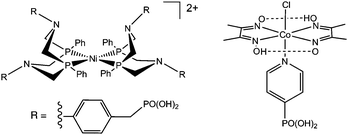
Herein, we have used a water-soluble Ni bis(diphosphine) catalyst (NiP),14 as well as a cobaloxime (CoP)17 (Scheme 1) to study inhibition of catalytic proton reduction activity by O2 and CO. Inhibition prevents catalysts from undergoing redox reactions essential for catalytic H2 evolution, therefore electrochemical analysis was fundamental to this work. Cyclic voltammetry has been used to monitor changes in the redox and electrocatalytic activity of CoP and NiP under atmospheres of O2 or CO on a short time-scale and controlled potential electrolysis (CPE) combined with H2 analysis has explored the inhibition of H2-evolution activity over longer periods of time. Spectroelectrochemistry allowed the potential-dependent formation of inhibited species to be analysed.
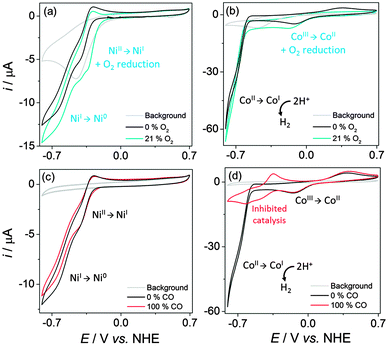
Cyclic voltammograms (CVs) were recorded on a glassy carbon disk electrode (0.07 cm2) at 100 mV s−1 using conditions optimised for high catalyst activity (pH 4.5 for NiP14 and pH 7 for CoP17,18). Initial studies into H2 inhibition (up to 100% H2) showed no product inhibition for NiP and CoP (Fig. S1, ESI†), allowing the effect of other inhibiting gases to be established during proton reduction. Fig. 1a and b display CVs of NiP and CoP under inert and aerobic atmospheres. Irreversible O2 reduction occurs at Ep = −0.5 V vs. normal hydrogen electrode (NHE) at the glassy carbon electrode, resulting in an increased current response in air, which must be taken into account in this analysis.
Under inert conditions, the CV of NiP displays two waves at potentials more negative than −0.3 V vs. NHE, which have been assigned to the reduction of NiII to NiI followed by NiI to a formal Ni0.14 The CV lacks a strong catalytic wave, presumably because most of the proton reduction by NiP occurs after Ni0 has diffused away from the electrode–solution interface.19 The CV trace recorded under 21% O2 (blue trace in Fig. 1a) shows almost no change compared to inert conditions when the O2 reduction current (grey trace) is disregarded. The degree of inhibition could not be obtained from the CVs due to the weak catalytic wave of NiP. CPE subsequently confirmed the catalytic proton reduction activity of NiP and was used to monitor the degree of O2 inhibition (see below).
The analogous CVs of CoP are displayed in Fig. 1b. Under inert conditions, the cobaloxime first undergoes a reduction from CoIII to CoII, followed by a strong catalytic wave at an onset potential of −0.6 V, as CoII is reduced to CoI and proton reduction catalysis is initiated.20 Under 21% O2, the proton reduction wave of CoP is almost identical suggesting high catalytic activity under air. The O2 reduction wave at −0.5 V however overlaps with the CoIII/CoII reduction wave around −0.2 V vs. NHE suggesting that CoII may be reducing dissolved O2. Catalytic H2 generation is thus in competition with oxidation of the reduced Co species (CoII and CoI) by O2. This was confirmed through analysis of the CoIII/CoII redox couple in air, which showed a loss of the anodic CoII to CoIII wave due to prior oxidation of CoII by O2 (Fig. S2, ESI†).21,22
CVs of NiP and CoP under a CO atmosphere are presented in Fig. 1c and d. Assuming saturation of water with CO at a concentration of 1 mM,23 the concentration of CO is comparable to the catalyst concentration. The reduction waves of NiP do not show any significant changes upon introduction of 100% CO (Fig. 1c). The cobaloxime demonstrates a low tolerance towards CO compared to the Ni bis(diphosphine) catalyst (see results from CPE below). CVs of CoP under N2 and CO have identical CoIII/CoII reduction (Ep = −0.14 V) and oxidation (Ep = +0.4 V) waves under N2 and CO (Fig. 1d). Upon reduction of CoII to CoI however, the proton reduction activity is no longer observed as the cobaloxime is inhibited.
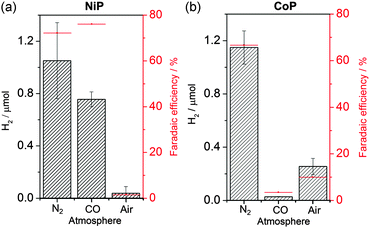
A long-term, more quantitative measure of inhibition was achieved through CPE, which analysed changes in the H2 produced by both catalysts. CPE was particularly important for the study of NiP, where little catalysis was observed in the CVs. A glassy carbon rod (approximately 2 cm2) was held at −0.4 V vs. NHE for NiP and −0.7 V vs. NHE for CoP, whilst stirring under different atmospheres. The H2 produced was detected by headspace gas chromatography (Fig. 2 and Table S1, ESI†). Faradaic efficiencies were calculated and gave respectable numbers for molecular catalysts held at such low overpotentials (>65% in all cases).
CPE of CoP for 15 min in the presence of air illustrated the tolerance of cobaloximes to O2. The CPE timescale was kept short to avoid the formation of heterogeneous catalysts on the electrode surface.24 A drop in proton reduction activity was seen under air compared to N2, due to increasing catalyst oxidation by O2, yet the catalyst still retained appreciable activity. The Faradaic efficiency similarly drops due to increasing O2 reduction by both the electrode and catalyst. The remarkable tolerance towards O2 has been attributed previously to the abundance of aqueous protons over O2 in the electrochemical cell10 (0.3 mM O2 under aerobic conditions) combined with the low affinity of the cobaloxime for forming irreversible inhibition products with O2. The reduction of oxygen presumably leads to the production of water in a similar manner to oxygen tolerant hydrogenases,25 allowing parallels to be drawn between these systems.
The activity of NiP was much more sensitive to O2. Despite the apparent tolerance displayed in the CV (Fig. 1a), 60 min of CPE under air at −0.4 V vs. NHE produced only negligible amounts of H2. The level of H2 recorded was comparable to the small quantity produced by the glassy carbon rod electrode without a catalyst. This complete inhibition of NiP suggests that an oxidised, inactive inhibition product is forming. Studies into O2 reduction by similar structures identified the formation of inactive phosphine oxides at low Ni oxidation states26 and gives a possible explanation for the observed inhibition on the CPE timescale. It may thus be concluded that in order to prevent O2 inhibition it is important to avoid ligand functionality that is susceptible to irreversible oxidation, such as phosphines. Upon repurging with N2 72% of the initial H2 production rate was observed, as the catalyst molecules that are not reduced during CPE are relatively O2 stable in the bulk solution.
On the other hand, NiP is completely tolerant to CO. CPE produced similar levels of H2 under both 100% CO and 100% N2 (Fig. 2a). The tolerance of NiP towards CO is remarkable considering the strongly inhibitive effect of CO on most catalytic surfaces, such as Pt. Experiments into Pt inhibition showed that the H2 produced by a Pt disk electrode held at −0.4 V vs. NHE for 15 min produced minimal H2 under CO (Fig. S3 and Table S2, ESI†). The Ni bis(diphosphine) structure is designed to mimic hydrogenase enzymes27 and the coordination sphere of a similar Ni bis(diphosphine) complex has previously demonstrated rapid reversible CO binding.28 This may prevent the CO from having a significant inhibiting impact on proton reduction in a manner much akin to the few reported CO-tolerant hydrogenases.29,30
This result is in contrast to CoP, which exposes an easily accessible coordination site in its catalytic cycle,31 and is consequently susceptible to CO binding. CoP was completely inhibited by CO; 15 min of CPE at −0.7 V vs. NHE produced minimal H2. However, CO inhibition of CoP was completely reversible and 100% of the electroactivity could be regained after purging with N2 (see Fig. S4, ESI†). The inhibition of the aforementioned Pt disk was irreversible and could not be reactivated with a N2 purge (Fig. S3, ESI†).
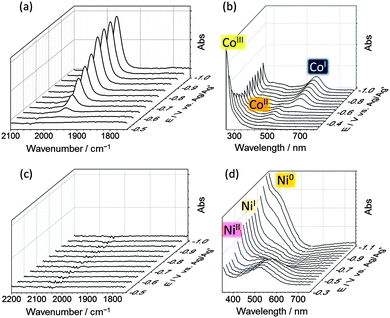
IR-spectroelectrochemical studies were carried out to gain a better understanding of cobaloxime inhibition. Using a spectroelectrochemical cell (Pt working and counter electrodes, Ag wire reference)32 IR-spectra were taken of [CoCl(dimethylglyoximato)2(4-methoxypyridine)] under CO at a range of potentials (Fig. 3a). The methoxypyridine analogue of CoP was used due to its higher solubility in MeOH.31 UV-visible spectra were recorded to follow the oxidation state change of the complex (Fig. 3b).
Upon reaching potentials at which CoI forms in the UV/visible spectra33 (−0.65 V vs. Ag/Ag+) a peak is observed in the IR spectra at 1970 cm−1, which is the expected region for a cobaloxime-carbonyl species.34 This peak has been assigned to substitution of the labile axial pyridine for CO at the low Co oxidation state.35 No carbonyl peak was observed under an atmosphere of N2 (Fig. S5, ESI†). Electron withdrawing axial ligands, such as CO, decrease cobaloxime proton reduction activity by reducing the basicity of the intermediate Co–H that forms in the catalytic cycle,31 thereby explaining the loss of catalytic activity.
No Ni-carbonyl peaks are present in the IR spectra of NiP under a CO atmosphere at any potential applied (Fig. 3c). The UV-visible spectroelectrochemistry displays bands that have been assigned to NiII/NiI/Ni0 from −0.4 to −1 V vs. Ag/Ag+ (Fig. 3d). The NiII state has a band at 520 nm corresponding to a pink color that is lost upon formation of NiI. The NiI state shows little absorption in the visible region but a shift in the UV peak at 250 nm occurs (Fig. S6, ESI†). Upon formation of Ni0 a yellow color is seen as suggested by the shoulder in the UV-vis spectrum at 400 nm and previous accounts.36 The lack of Ni-carbonyl peak across these oxidation states illustrates the tolerance of the Ni bis(diphosphine) to carbonyl binding and explains the sustained proton reduction activity under these conditions.
In summary, Ni bis(diphosphine) and cobaloxime catalysts are widely used state-of-the-art catalysts for the reduction of aqueous protons. Our study demonstrates their distinct tolerance to well-known gaseous inhibitors and illustrates the ways in which molecular catalysts can be designed to fulfill the requirements of a specific system. NiP shows unprecedented activity under CO and can therefore be employed in systems where CO is present, such as syngas generating devices. CO reversibly inhibits CoP due to the formation of an inactive Co–CO species as confirmed by IR-spectroelectrochemistry. On the other hand, the cobaloxime showed appreciable tolerance towards O2, whereas the Ni bis(diphosphine) complex lost all activity. Ongoing studies seek to gain a more detailed understanding of the relationship between the structure of a catalyst and its resultant tolerance to inhibition.
Financial support from the EPSRC (EP/H00338X/2), the Christian Doppler Research Association (Austrian Federal Ministry of Science, Research and Economy and National Foundation for Research, Technology and Development), and the OMV Group is gratefully acknowledged.
Notes and references
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed.
- M. E. Dry, Catal. Today, 2002, 71, 227–241 CrossRef CAS.
- P. Du and R. Eisenberg, Energy Environ. Sci., 2012, 5, 6012–6021 CAS.
- N. P. Dasgupta, C. Liu, S. Andrews, F. B. Prinz and P. Yang, J. Am. Chem. Soc., 2013, 135, 12932–12935 CrossRef CAS PubMed.
- E. Reisner, Eur. J. Inorg. Chem., 2011, 1005–1016 CrossRef CAS.
- F. A. Armstrong, N. A. Belsey, J. A. Cracknell, G. Goldet, A. Parkin, E. Reisner, K. A. Vincent and A. F. Wait, Chem. Soc. Rev., 2009, 38, 36–51 RSC.
- T. S. Teets and D. G. Nocera, Chem. Commun., 2011, 47, 9268–9274 RSC.
- W. T. Eckenhoff and R. Eisenberg, Dalton Trans., 2012, 41, 13004–13021 RSC.
- H. I. Karunadasa, E. Montalvo, Y. Sun, M. Majda, J. R. Long and C. J. Chang, Science, 2012, 335, 698–702 CrossRef CAS PubMed.
- F. Lakadamyali, M. Kato, N. M. Muresan and E. Reisner, Angew. Chem., Int. Ed., 2012, 51, 9381–9384 CrossRef CAS PubMed.
- B. Mondal, K. Sengupta, A. Rana, A. Mahammed, M. Botoshansky, S. G. Dey, Z. Gross and A. Dey, Inorg. Chem., 2013, 52, 3381–3387 CrossRef CAS PubMed.
- J. G. Kleingardner, B. Kandemir and K. L. Bren, J. Am. Chem. Soc., 2014, 136, 4–7 CrossRef CAS PubMed.
- M. L. Helm, M. P. Stewart, R. M. Bullock, M. Rakowski DuBois and D. L. DuBois, Science, 2011, 333, 863–866 CrossRef CAS PubMed.
- M. A. Gross, A. Reynal, J. R. Durrant and E. Reisner, J. Am. Chem. Soc., 2014, 136, 356–366 CrossRef CAS PubMed.
- A. Dutta, S. Lense, J. Hou, M. H. Engelhard, J. A. S. Roberts and W. J. Shaw, J. Am. Chem. Soc., 2013, 135, 18490–18496 CrossRef CAS PubMed.
- J. R. McKone, N. S. Lewis and H. B. Gray, Chem. Mater., 2014, 26, 407–414 CrossRef CAS.
- F. Lakadamyali and E. Reisner, Chem. Commun., 2011, 47, 1695–1697 RSC.
- P. Du, K. Knowles and R. Eisenberg, J. Am. Chem. Soc., 2008, 130, 12576–12577 CrossRef CAS PubMed.
- C. Costentin, S. Drouet, M. Robert and J.-M. Savéant, J. Am. Chem. Soc., 2012, 134, 11235–11242 CrossRef CAS PubMed.
- J. L. Dempsey, B. S. Brunschwig, J. R. Winkler and H. B. Gray, Acc. Chem. Res., 2009, 42, 1995–2004 CrossRef CAS PubMed.
- M. Shamsipur, A. Salimi, H. Haddadzadeh and M. F. Mousavi, J. Electroanal. Chem., 2001, 517, 37–44 CrossRef CAS.
- G. N. Schrauzer, Angew. Chem., Int. Ed., 1976, 15, 417–426 CrossRef CAS PubMed.
- P. Scharlin, R. Battino, E. Silla, I. Tuñón and J. L. Pascual-Ahuir, Pure Appl. Chem., 1998, 70, 1895–1904 CrossRef CAS.
- S. Cobo, J. Heidkamp, P.-A. Jacques, J. Fize, V. Fourmond, L. Guetaz, B. Jousselme, V. Ivanova, H. Dau, S. Palacin, M. Fontecave and V. Artero, Nat. Mater., 2012, 11, 802–807 CrossRef CAS PubMed.
- W. Lubitz, H. Ogata, O. Rüdiger and E. Reijerse, Chem. Rev., 2014, 114, 4081–4148 CrossRef CAS PubMed.
- J. Y. Yang, R. M. Bullock, W. G. Dougherty, W. S. Kassel, B. Twamley, D. L. DuBois and M. Rakowski DuBois, Dalton Trans., 2010, 39, 3001–3010 RSC.
- A. D. Wilson, R. H. Newell, M. J. McNevin, J. T. Muckerman, M. Rakowski DuBois and D. L. DuBois, J. Am. Chem. Soc., 2006, 128, 358–366 CrossRef CAS PubMed.
- A. D. Wilson, K. Fraze, B. Twamley, S. M. Miller, D. L. DuBois and M. Rakowski DuBois, J. Am. Chem. Soc., 2008, 130, 1061–1068 CrossRef CAS PubMed.
- K. A. Vincent, J. A. Cracknell, O. Lenz, I. Zebger, B. Friedrich and F. A. Armstrong, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 16951–16954 CrossRef CAS PubMed.
- X. Luo, M. Brugna, P. Tron-Infossi, M. T. Giudici-Orticoni and É. Lojou, J. Biol. Inorg. Chem., 2009, 14, 1275–1288 CrossRef CAS PubMed.
- D. W. Wakerley and E. Reisner, Phys. Chem. Chem. Phys., 2014, 16, 5739–5746 RSC.
- M. Krejčik, M. Daněk and F. Hartl, J. Electroanal. Chem., 1991, 317, 179–187 CrossRef.
- N. M. Muresan, J. Willkomm, D. Mersch, Y. Vaynzof and E. Reisner, Angew. Chem., Int. Ed., 2012, 51, 12749–12753 CrossRef CAS PubMed.
- X. Hu, B. M. Cossairt, B. S. Brunschwig, N. S. Lewis and J. C. Peters, Chem. Commun., 2005, 4723–4725 RSC.
- T. M. McCormick, Z. Han, D. J. Weinberg, W. W. Brennessel, P. L. Holland and R. Eisenberg, Inorg. Chem., 2011, 50, 10660–10666 CrossRef CAS PubMed.
- E. S. Wiedner, J. Y. Yang, S. Chen, S. Raugei, W. G. Dougherty, W. S. Kassel, M. L. Helm, R. M. Bullock, M. Rakowski DuBois and D. L. DuBois, Organometallics, 2012, 31, 144–156 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, Tables S1 and S2 and Fig. S1–S6. See DOI: 10.1039/c4cc06159d |
| This journal is © The Royal Society of Chemistry 2014 |