Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluorescence lifetime-based sensing of sodium by an optode†
Thomas
Schwarze
a,
Holger
Müller
a,
Sandra
Ast
ac,
Dörte
Steinbrück
b,
Sascha
Eidner
b,
Felix
Geißler
b,
Michael U.
Kumke
*b and
Hans-Jürgen
Holdt
*a
aUniversity of Potsdam, Inorganic Chemistry, Karl-Liebknecht-Straße 24-25, 14476 Golm, Germany. E-mail: holdt@uni-potsdam.de; Fax: +49 331 977 5055
bUniversity of Potsdam, Physical Chemistry, 14476 Golm, Germany. E-mail: kumke@uni-potsdam.de
cUniversity of Sydney, School of Chemistry, NSW2006, Sydney, Australia
First published on 19th September 2014
Abstract
We report a 1,2,3-triazol fluoroionophore for detecting Na+ that shows in vitro enhancement in the Na+-induced fluorescence intensity and decay time. The Na+-selective molecule 1 was incorporated into a hydrogel as a part of a fiber optical sensor. This sensor allows the direct determination of Na+ in the range of 1–10 mM by measuring reversible fluorescence decay time changes.
Sodium plays a crucial role in many physiological processes and the Na+ concentration largely differs in intra- and extracellular compartments. The concentration of intracellular free Na+ is in the range of 5–30 mM, whereas that of extracellular is more than 100 mM.1 For the determination and visualisation of intra- or extracellular Na+ levels, Na+-responsive fluorescent probes are suitable tools. Minta and Tsien designed the first fluorescent indicators for detecting cytosolic Na+ (∼30 mM) with dissociations constants Kd < 50 mM.1 Several Na+-sensitive fluorescent probes are commercially available, e.g. SBFI (sodium-binding benzofuran isophthalate) (Kd ∼ 11 mM),1,2 Sodium Green (Kd ∼ 21 mM),2 CoroNa Green (Kd ∼ 80 mM)2,3a and CoroNa Red (Kd ∼ 200 mM).2,3b Furthermore, photoinduced electron transfer (PET)-type fluoroionophores have been proven to be successful as highly selective sodium sensing molecules.4–6 For the analysis of sodium in serum and whole blood (∼140 mM) He et al. designed a PET-fluoroionophore which consists of a N-(o-methoxyphenyl)aza-15-crown-5 ionophore (Kd ∼ 80 mM) and a 4-aminonaphthalimid fluorophore.4 An application of this fluoroionophore is the use in clinical diagnosis as a part of optical sensors for analytes such as Na+ in whole blood.7 For the continuous sensing of Na+, several optodes based on fluorescence intensity changes have been developed (e.g., to be used in films or beads).8a–n Many of these optode layouts are based on a general concept, in which the detection of the targeted analyte and the optical signal generation are separated. The analyte (here Na+) is bound and induces an ion exchange which subsequently is detected by a pH-sensitive dye. The advantage of such an approach is that the optical part of the sensor can be applied to many different analytes. However, the optodes can be cross-sensitive to pH changes in the samples. To date, there has been no fiber-optical sensor for direct Na+ sensing (e.g., on the basis of a PET-fluoroionophore) incorporated in a hydrogel using fluorescence decay time changes as the detection principle.
Sensor endurance is also a major challenge in general.9 Concepts often rely on the ratiometric measurements of intensities or are based on fluorescence decay time determination. These two sensing schemes are independent of the probe concentration and aging effects of the optode (e.g., due to photobleaching). For monovalent ions such as sodium and potassium, it is difficult to develop ratiometric fluoroionophores,10 and only a few fluorescence decay time based probes for Na+ are available, such as Sodium Green.11
There is a fast increasing demand for tailored fluorescence probes (with respect to e.g., the spectral range, analyte or photostability) in life sciences. Here, among others, sodium is one of the top analytes and the development of a novel fluorescent probe based on the detection of changes in the fluorescence decay time is desirable. Developing a sensing scheme for the direct detection of sodium based on fluorescence decay time measurements will not only minimize the cross-sensitivity for pH and enhance the overall performance of the sensor with respect to aging effects but will also add another dimension (decay time in addition to spectral parameters) to the analysis, which will be beneficial for the development of multianalyte sensing schemes. Overall, the development of new optical sensor devices with (a) suitable Kd values for extracellular/blood or intracellular sodium measurements, (b) an excellent selectivity over physiological potassium concentrations, (c) no pH interference in the physiological pH range, (d) a fast response time, (e) a reversible signal change, and (f) a change of the fluorescence decay time is the focus of attention at present.
Recently, we found that in CuAAC (Cu(I)-catalyzed 1,3-dipolar azide alkyne cycloaddition) reaction generated fluoroionophores, the electronic conjugation of a N-phenylaza-18-crown-612a or of a N-(2-methoxyethoxyphenyl) aza-18-crown-612b ionophore and a 7-diethylaminocoumarin fluorophore through a 1,2,3-triazol-1,4-diyl π-linker results in a perfect signal transduction chain for the exclusive sensing of K+ under simulated physiological conditions.12a,b Thereby, we prepared a fluorescent membrane sensor by incorporating the N-(2-methoxyethoxyphenyl)aza-18-crown-6-fluoroionophore into a hydrogel, which showed fast and fully reversible fluorescence intensity changes in the range of 1–10 mM potassium.12b
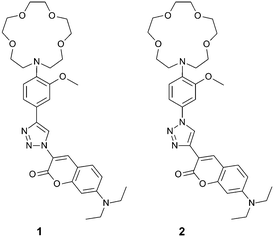
In this communication, we have maintained the signal transduction chain: aniline–triazole–coumarin in the construction of Na+-sensitive PET-fluoroionophores 1 and 2 (Scheme 1). Thus, for selective Na+ binding the N-(o-methoxyphenyl)aza-15-crown-5 ionophore4,6d was selected owing to its excellent selectivity for Na+ over K+ in the range of 100–180 mM. Herein, in Tris buffer Na+ induces fluorescence intensity and decay time enhancement in 1 or 2. When 1 was embedded in the hydrogel poly(2-hydroxypropyl)methacrylate (PHPMA), we found a reversible change in the decay-time and intensity, obtained from fiber-optical fluorescence measurements. To the best of our knowledge, it is the first fiber-optical sensor for Na+ sensing based on a combination of a PET-fluoroionophore and of the PHPMA hydrogel using fluorescence decay time changes as the fundamental detection principle.
The CuAAC of the ethynyl-functionalized N-(o-methoxyphenyl)aza-15-crown-5 with 3-azido-7-diethylaminocoumarin13a afforded 1,2,3-triazol fluoroionophore 1. The corresponding constitutional isomer 2 was obtained by the reaction of the azido-functionalized N-(o-methoxyphenyl)aza-15-crown-5 with 3-ethynyl-7-diethylaminocoumarin13b (see ESI†).
The UV-Vis-absorption spectra of 1 and 2 in Tris buffer (10 mM, pH = 7.2) show the typical long-wavelength coumarin CT absorption band at λmax = 422 nm (1) and 419 nm (2) (Fig. S1a and b, ESI†). The isomeric 1,2,3-triazol-1,4-diyl linkage has only a small effect on λmax. In the UV-Vis-absorption spectra of 1 and 2 a second CT absorption band at ∼280 nm (1) and ∼315 nm (2) is observed (Fig. S1, ESI†) which is typical for π-conjugated 1,2,3-triazol-1,4-diyl-fluoroionophores.12a,b The lowest energy coumarin absorption bands at 422 nm for 1 and 419 nm for 2 are essentially unchanged upon Na+ or K+ addition (Fig. S1a and b, ESI†). The coordination of Na+ in the cavity of the aza-15-crown-5 unit can be seen from the short-wavelength CT transition. The binding of Na+ reduces the electron donating character of the anilino unit which leads to a decrease of the CT absorption band at ∼280 nm (1) and ∼315 nm (2) (Fig. S1, ESI†).
As already observed for N-phenylaza-18-crown-6 and N-(2-methoxyethoxyphenyl)aza-18-crown-6 substituted 1,2,3-triazol-coumarin fluoroionophores,12a,b the fluorescence of the coumarin unit is almost completely quenched. The fluorescence quantum yields of 1 and 2 in Tris buffer are 0.009 and 0.051, respectively. Probably, a reductive PET occurs from the anilino-triazole unit to the coumarin moiety (see ESI†).
We investigated the influence of Na+ and K+ on the fluorescence of 1 and 2 in the physiological relevant concentration range of 0–180 mM Na+ or K+ (see the ESI† for K+). Fig. 1a shows the fluorescence enhancement of 1 in a buffered H2O/DMSO mixture (99/1, v/v; 10 mM Tris; pH = 7.2) in the presence of 0–180 mM Na+. We observed Na+-induced fluorescence enhancements of 1 (FEFNa+: 5.0 ± 0.1) and 2 (FEFNa+: 2.5 ± 0.1). The fluorescence signals of 1 and 2 are slightly effected by K+ (FEFK+: 1.5 ± 0.1, Table S1, ESI†). The FEF of 1 in the presence of 180 mM Na+ is higher than of 2 (see Table S1, ESI†). A Na+-induced fluorescence enhancement of 1 (FEF: 4.7 ± 0.1) and 2 (FEF: 2.6 ± 0.1) is also observed in the presence of physiological concentrations of K+ ([K+] + [Na+] = 180 mM). Furthermore, Benesi–Hildebrand plots for Na+ binding show a linear relationship (R2 = 0.998 (1), Fig. S5a, ESI† and R2 = 0.9986 (2), Fig. S5b, ESI†), indicating 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation between 1 or 2 and Na+. The Kd values were calculated from the fluorescence titration curves. The Kd value of 1 for Na+ is ∼120 mM (Fig. S5a and S7a, ESI†) and for 2 we found a higher Kd value of ∼260 mM (Fig. S5b and S7b, ESI†), suggesting that the Kd is influenced by the isomeric triazole linkage.
1 complexation between 1 or 2 and Na+. The Kd values were calculated from the fluorescence titration curves. The Kd value of 1 for Na+ is ∼120 mM (Fig. S5a and S7a, ESI†) and for 2 we found a higher Kd value of ∼260 mM (Fig. S5b and S7b, ESI†), suggesting that the Kd is influenced by the isomeric triazole linkage.
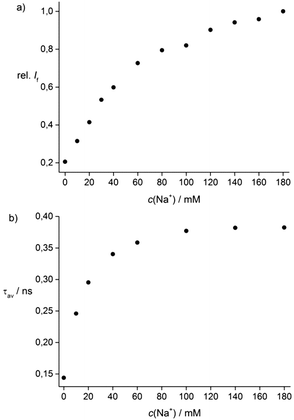 | ||
| Fig. 1 Titration curves of 1 (c = 10−5 M, λex = 422 nm) in 10 mM Tris buffer (pH = 7.2) + Na+ based on (a) fluorescence intensity at 500 nm and (b) fluorescence decay time measurements (see ESI†). | ||
The selectivity of 1 for Na+ over K+ in the physiological range is shown in Fig. S3c (ESI†). The Kd value of 1 for K+ is ∼276 mM and of 2 for K+ is ∼1060 mM (Fig. S6a and b, ESI†), indicating that 1 is ∼3-fold more selective for Na+ than for K+ and 2 ∼4-fold more selective.
To investigate the pH-sensitivity of 1 and 2, the fluorescence intensity was measured in H2O at different pH values (see ESI†). The resulting pKa of 1 is 4.9 and that of 2 is 4.6, respectively. 1 and 2 are unaffected in the physiological relevant pH range of 6–8 (Fig. S8, ESI†).
Due to the underlying PET process in 1, the observed fluorescence kinetics are complex. The fluorescence decay curves at λem = 500 nm could be reasonably well fitted using a bi-exponential decay kinetics yielding main decay times of 0.08 ns (90%) for 1 and 0.39 ns (99%) for 1 + 180 mM Na+ (Table S1, ESI†). During complexation of Na+ the fraction of the short fluorescence decay time (0.08 ns) component decreases and the average fluorescence decay time increases (Fig. 1b) as the fraction of the longer fluorescence decay time increases. The Kd value of 1 + Na+ calculated based on the average fluorescence decay time is ∼15 mM (see ESI†). The fluorescence decay times of 1 were only slightly affected by K+ ions (see Table S1, ESI†). Furthermore, we observed for 2 + Na+ a similar decay time behaviour as found for 1 + Na+ (Table S1, ESI†).
Probe 1 was selected for studies on its application as a Na+-sensor because it showed a higher Na+/K+ fluorescence response than probe 2. To apply 1 in a sensor membrane for continuous monitoring of Na+ concentrations in liquid streams, such as blood, we incorporated it into a polymer matrix. The PHPMA hydrogel is a convenient polymer for making stable membrane sensors.14a,b For immobilisation of 1 within the hydrogel, both were dissolved in EtOH. This homogeneous mixture was used for film and optode preparation (see ESI†). Firstly, we prepared films of the mixture on glass slides, which we placed in standard cuvettes. The concentration of 1 in these films was 3.75 × 10−5 M. In order to avoid difficulties inherent to fluorescence intensity based measurements (vide supra), we determined Na+-induced fluorescence decay time changes by time-correlated single photon counting (TCSPC). In the hydrogel we found a fluorescence decay time τf of 2.1 ns for 1, which is distinctly longer than that observed in solution (see Table S1, ESI†). In solution the decay time is faster than that in the fixed polymer, because the rate constant of quenching by radiationless processes is larger. The decay time τf increases from 2.1 ns in 10 mM Tris buffer to 2.7 ns in the presence of 140 mM Na+. The Kd value determined by TCSPC measurements of 1 for Na+ is 42 mM in the PHPMA polymer (see Fig. S12, ESI†).
In the next step we investigated the τf behaviour of 1 in a PHPMA film using a low-cost frequency-domain spectrometer (FD-S) suitable for in situ applications. Therefore, we prepared a PHPMA-based film with a higher dye concentration of 1 (c = 7.5 × 10−4 M) which is needed for the FD-S set-up due to sensitivity requirements. We found decay times for 1 (τf = 2.2 ns) and 1 + 100 mM Na+ (τf = 2.9 ns) in PHPMA by FD-S (see Fig. S13, ESI†) comparable to the TCSPC measurements, but the Kd value of 1 + Na+ decreases to 1.6 mM. Due to the higher dye concentration in the polymer matrix the sensitivity to sodium seemed to increase. The Kd value of 1 + K+ in PHPMA is 6355 mM (Fig. S13, ESI†) indicating the highly improved Na+/K+ selectivity of the sensor film.
Finally, in order to further develop this outstanding Na+-selective film into an optode for fiber-optical measurements (Fig. S10 and S11, ESI†), fluorescence intensity and decay time measurements were successfully carried out. The fluorescence intensity increases upon dipping the optode in Na+ solutions of different concentrations up to 50 mM (see Fig. 2a). The Kd value of 1 + Na+ based on intensity changes determined by the optode is 6 mM. Comparable results were obtained by using FD-S. Here, the Kd value of 1 + Na+ is 2.4 mM using this fiber-optical sensor (see Fig. 2b). Consequently, this optode allows determination of Na+ by fluorescence decay time measurements in the range of 1–10 mM.
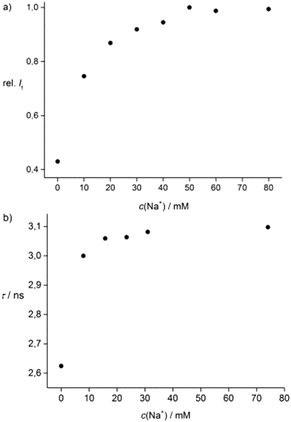 | ||
| Fig. 2 Titration curves of 1 (c = 7.5 × 10−4 M) in PHPMA + Na+ based on (a) fluorescence intensity and (b) FD-S measurements by an optode. | ||
Furthermore, for continuous sensing of Na+ in the range of 1–10 mM by measuring fluorescence intensity and decay time changes of this optode we tested the reversibility. As a proof of principle our first results for continuous Na+ sensing are shown in Fig. S17 (ESI†). The response time of the optode after switching the solution from 0 mM Na+ to 10 mM Na+ during a time period of 2 h shows a good reversible behaviour. The response time depends on the direction of the change: by increasing the sodium concentration the response is faster (<8 min) than by decreasing the concentration (15 min). Here, the layout of the optode (the thickness of the polymer and the concentration of the probe) is a key parameter in this respect and will be further improved in order to reduce the response time.
In summary, we have synthesised two regioisomeric fluoroionophores 1 and 2 for Na+ by “click” chemistry. In solution they showed different Kd values (1 + Na+ ∼120 mM and 2 + Na+ ∼260 mM) under simulated physiological conditions. Probe 1 is a capable fluoroionophore for the selective determination of extracellular Na+ levels by measuring fluorescence intensity changes. Further, 1 was incorporated into a membrane sensor consisting of a polymethacrylate hydrogel and, in this form, it enabled the continuous monitoring of Na+ (1–10 mM) with a reversible response based on fluorescence decay time measurements. Overall, we have shown the first reversible fiber-optical sensor for Na+ ions utilizing fluorescence decay time changes. Further studies to improve the brightness, photostability, Na+-induced overall fluorescence decay time changes and the response time of our optode are currently underway in our laboratories.
Notes and references
- A. Minta and R. Tsien, J. Biol. Chem., 1989, 264, 19449 CAS.
- The Molecular Probes® Handbook—A Guide to Fluorescent Probes and Labeling Technologies, Molecular Probes, Eugene, OR, 11th edn, 2005 Search PubMed.
- (a) V. V. Martin, A. Rothe and K. R. Gee, Bioorg. Med. Chem. Lett., 2005, 15, 1851 CrossRef CAS PubMed; (b) V. V. Martin, A. Rothe, Z. Diwu and K. R. Gee, Bioorg. Med. Chem. Lett., 2004, 14, 5313 CrossRef CAS PubMed.
- H. He, M. A. Mortellaro, M. J. P. Leitner, S. T. Young, R. J. Fraatz and J. K. Tusa, Anal. Chem., 2003, 75, 549 CrossRef CAS.
- J. F. Callan, A. P. De Silva and D. C. Magri, Tetrahedron, 2005, 61, 8551 CrossRef CAS PubMed.
- (a) A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson and M. Nieuwenhuizen, Chem. Commun., 1996, 1967 RSC; (b) M. K. Kim, C. S. Lim, J. T. Hong, J. H. Han, H.-Y. Jang, H. M. Kim and B. R. Cho, Angew. Chem., Int. Ed., 2010, 49, 364 CrossRef CAS PubMed; (c) A. R. Serker, C. H. Heo, M. Y. Park, H. W. Lee and H. M. Kim, Chem. Commun., 2014, 50, 1309 RSC; (d) T. Gunnlaugsson, M. Nieuwenhuyzen, L. Richard and V. Thoss, J. Chem. Soc., Perkin Trans. 2, 2002, 141 CAS.
- J. K. Tusa and H. He, J. Mater. Chem., 2005, 15, 2640 RSC.
- (a) J. M. Dubach, D. I. Harjes and H. A. Clark, J. Am. Chem. Soc., 2007, 129, 8418 CrossRef CAS PubMed; (b) X. Yang, K. Wang, D. Xiao, C. Guo and Y. Xu, Talanta, 2000, 52, 1033 CrossRef CAS; (c) C. Yang, T. Liu, Y. Xu and Y. Qin, Sens. Actuators, B, 2014, 192, 423 CrossRef CAS PubMed; (d) W. H. Chan, A. W. M. Lee, Y. S. Lam and J. Z. Lu, Microchem. J., 2002, 72, 201 CrossRef CAS; (e) G. Mistlberger, X. Xie, M. Pawlak, G. A. Crespo and E. Bakker, Anal. Chem., 2013, 85, 2983 CrossRef CAS PubMed; (f) L. Xie, Y. Qin and H.-Y. Chen, Anal. Chem., 2012, 84, 1969 CrossRef CAS PubMed; (g) G. A. Crespo and E. Bakker, Analyst, 2012, 137, 4988 RSC; (h) K. Wygladacz and E. Bakker, Anal. Chim. Acta, 2005, 532, 61 CrossRef CAS PubMed; (i) K. Kurihara, K. Nakamura, E. Hirayama and K. Suzuki, Anal. Chem., 2002, 74, 6323 CrossRef CAS; (j) J. S. Benco, H. A. Nienaber and W. Grant McGimpsey, Sens. Actuators, B, 2002, 85, 126 CrossRef CAS; (k) X. Yang, K. Wang and C. Guo, Anal. Chim. Acta, 2000, 407, 45 CrossRef CAS; (l) K. Kurihara, M. Ohtsu, T. Yoshida, T. Abe, H. Hisamoto and K. Suzuki, Anal. Chem., 1999, 71, 3558 CrossRef CAS; (m) W. H. Chan, A. W. M. Lee, D. W. J. Kwong, Y.-Z. Liang and K.-M. Wang, Analyst, 1997, 122, 657 RSC; (n) F. Buchholz and N. Buschmann, Sens. Actuators, B, 1992, 9, 41 CrossRef CAS.
- O. S. Wolfbeis, Angew. Chem., Int. Ed., 2013, 52, 9864 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, New York, 2006 Search PubMed.
- H. Szmacinski and J. R. Lakowicz, Anal. Biochem., 1997, 250, 131 CrossRef CAS PubMed.
- (a) S. Ast, H. Müller, R. Flehr, T. Klamroth, B. Walz and H.-J. Holdt, Chem. Commun., 2011, 47, 4685 RSC; (b) S. Ast, T. Schwarze, H. Müller, A. Sukhanov, S. Michaelis, J. Wegener, O. S. Wolfbeis, T. Körzdörfer, A. Dürkop and H.-J. Holdt, Chem. – Eur. J., 2013, 19, 14911 CrossRef CAS PubMed.
- (a) K. Sivakumar, F. Xie, B. M. Cash, S. Long, H. N. Barnhill and Q. Wang, Org. Lett., 2004, 6, 4603 CrossRef CAS PubMed; (b) D.-N. Lee, G.-J. Kim and H.-J. Kim, Tetrahedron Lett., 2009, 50, 4766 CrossRef CAS PubMed.
- (a) H. H. Chu and D. C. Fu, Macromol. Rapid Commun., 1998, 19, 107 CrossRef CAS; (b) D. Steinbrück, Faseroptische Sauerstoff-und pH-Sensorik mittels Phasenmodulationsspektroskopie, PhD thesis, Dissertation Universität, Potsdam, 2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cc06112h |
| This journal is © The Royal Society of Chemistry 2014 |