 Open Access Article
Open Access ArticleHaemoglobin wrapped covalently by human serum albumin mutants containing Mn(III) protoporphyrin IX: an O2 complex stable in H2O2 solution†
Y.
Daijima
and
T.
Komatsu
*
Department of Applied Chemistry, Faculty of Science and Engineering, Chuo University, 1-13-27 Kasuga, Bunkyo-ku, Tokyo 112-8551, Japan. E-mail: komatsu@kc.chuo-u.ac.jp
First published on 3rd September 2014
Abstract
We describe the synthesis of a haemoglobin (Hb) wrapped covalently by recombinant human serum albumin mutants [HSA(Y161H)] containing Mn(III) protoporphyrin IX (MnPP), the Hb–[HSA(Y161H)–MnPP]3 cluster, highlighting the formation of its O2-complex stable even in H2O2 solution.
Haemoglobin (Hb)-based O2 carriers (HBOCs) of several types have been developed in the last few decades as red blood cell (RBC) substitutes and as O2-therapeutic reagents,1–4 such as cross-linked Hb,5,6 polymerized Hb7,8 and (polyethylene glycol)-conjugated Hb.9,10 Nevertheless, the HBOCs gradually lose their O2-transporting capability by autoxidation of Hb to the ferric haem form (metHb) with the release of a superoxide (O2˙−) that is disproportionated to hydrogen peroxide (H2O2).11 In the RBC, superoxide dismutase (SOD) converts O2˙− to H2O2, which is then dismutated to H2O and O2 by catalase. Such protective enzyme systems are not present in the plasma. Therefore, these reactive oxygen species (ROS) produced by HBOCs cause not only tissue injury, but also further oxidation of Hb. In particular, H2O2 appears to be a potent species causing oxidative damage to Hb.12 Consequently, HBOCs with catalase activity are expected to be tremendously useful in practical applications. Chang et al. prepared a poly(Hb–SOD–catalase) hybrid and showed a significantly low metHb formation rate.13 This Hb–(antioxidant enzymes) conjugate demonstrated both O2-carrying and antioxidant properties.
More recently, we synthesized a covalent core–shell structured protein cluster composed of Hb at the centre and human serum albumins [wild type, HSA(wt)] at the periphery, Hb–HSA(wt)3, which is a structurally defined HBOC.14 Because HSA possesses only one free sulfhydryl group at Cys-34, we exploited a heterobifunctional cross-linker, N-succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) to combine the Cys-34 of HSA(wt) and the surface Lys ε-amino groups of Hb (Fig. 1). The major product formed is the Hb–HSA3 heterotetramer in a triangle form. From 3D reconstruction based on transmission electron microscopy images, the binding partners in Hb were identified as Lys-90(α1), Lys-82(β1) and Lys-16(α2).14
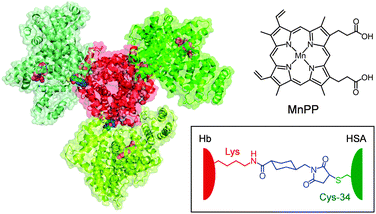 | ||
| Fig. 1 Structure model of the Hb–[HSA(Y161H)–MnPP]3 cluster (central Hb, red; peripheral HSAs, green). The haems (grey) in Hb and MnPPs (pinks) bound within subdomain IB of the exterior HSA(Y161H) units (see Fig. 2) are shown in a space-filling representation. The Cys-34 of HSA and the surface Lys of Hb were connected with a cross-linking agent (SMCC, blue). | ||
If one were able to confer antioxidant properties on the external HSA(wt) units of Hb–HSA(wt)3, then this construct would become a promising O2-carrier with high resistance against oxidation reactions. We chose the Mn(III) porphyrin as a potential candidate because it is an effective catalyst for H2O2 dismutation and because it lacks Fenton reaction-related toxicity.15 In this communication, we describe the synthesis of Hb wrapped covalently by recombinant HSA mutants containing Mn(III) protoporphyrin IX (MnPP, Fig. 1) and its markedly stable O2-complex in H2O2 solution. This super-structured haemoprotein possesses the dual functionalities of O2 transport and H2O2 dismutation.
The most prominent plasma protein in the bloodstream, HSA (Mw: 66.5 kDa), can bind widely diverse water-insoluble metabolites (fatty acids, bilirubin, thyroxin, etc.) and drugs (warfarin, diazepam, ibuprofen, etc.). This heart-shaped carrier protein consists of three similar domains (I–III), each of which includes two subdomains: A and B (Fig. 2A).16–18 Our crystallographic and spectroscopic studies revealed that Fe(III) protoporphyrin IX (FePP) is bound within a deep hydrophobic slot in subdomain IB of HSA(wt) with (i) a weak axial coordination of Tyr-161 (phenolate oxygen) to the central Fe ion and (ii) salt-bridges between the porphyrin propionates and basic amino acid residues at the pocket entrance (Arg-114, His-146 and Lys-190).19,20 We demonstrated previously that MnPP is also incorporated into this pocket of HSA(wt) with Tyr-161 coordination (Fig. 2B).21 Moreover, the recombinant HSA(Y161H) mutant, in which Tyr-161 is replaced with His by site-directed mutagenesis, similarly captures MnPP with an axial His-161 ligation to the central Mn ion.21 The HSA(Y161H)–MnPP complex displays characteristic split Soret bands at 374 and 473 nm and Q-bands at 559 and 594 nm (shoulder). They resemble those of the MnPP–His complex in MnPP-reconstituted myoglobin.22,23 However, the catalase activities of these manganese proteins have not been elucidated. We initially examined the H2O2 dismutation activities of the HSA(wt)–MnPP and HSA(Y161H)–MnPP complexes in phosphate buffered saline (PBS) solution by monitoring the generated O2 using an O2 electrode system. Immediately after addition of H2O2 to the HSA–MnPP solution, an increase in dissolved O2 was observed. With the coexistence of HSA alone, the O2 concentration did not change. We inferred that the catalase activities of HSA–MnPP complexes were attributable to the MnPP in the protein.
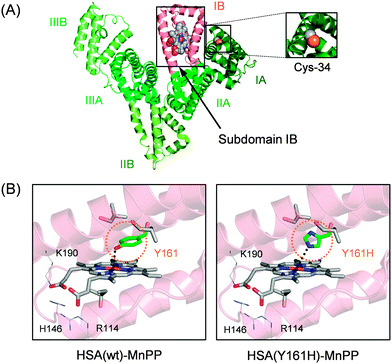 | ||
| Fig. 2 (A) Structure models of HSA–MnPP complex. (B) Schematic illustration of MnPP binding site in subdomain IB of HSA (prepared from PDB ID: 1O9X). In HSA(wt), Tyr-161 axially coordinates to the central Mn ion of MnPP. In HSA(Y161H), His-161 coordinates to the central Mn of MnPP. | ||
Then the steady-state kinetic parameters of the HSA–MnPP complexes were determined. A hyperbolic dependence of the initial rate (v0) on H2O2 concentration yielded the Michaelis constant (Km) and catalytic constant (kcat) (Table 1). It is noteworthy that the enzyme activity (kcat/Km) of HSA(Y161H)–MnPP becomes five-fold greater than that of HSA(wt)–MnPP. We can therefore conclude that the H2O2 dismutation activity is enhanced by replacing proximal Tyr with His, which has a strong electron-donating ability to the central Mn ion of MnPP.
| Catalase mimic | k cat (min−1) | K m (mM) | k cat/Km (M−1 min−1) |
|---|---|---|---|
| HSA(wt)–MnPP | 8 | 24 | 341 |
| HSA(Y161H)–MnPP | 39 | 24 | 1640 |
Next, we enwrapped Hb with recombinant HSA(Y161H) mutants to prepare a Hb–HSA(Y161H)3 cluster (see ESI†).14 The reaction mixture of SMCC-bound Hb and HSA(Y161H) includes Hb–HSA(Y161H)3 heterotetramer as the major compound. Using gel filtration chromatography (GFC), the main fraction was harvested (Fig. S1†) (yield: 80% based on Hb). The average HSA/Hb ratio was ascertained to be 3.0 using Hb and protein assays.
Subsequently, the ethanolic MnPP was added to the PBS solution of Hb–HSA(Y161H)3 (MnPP/cluster = 3.0, mol/mol) to create a Hb–[HSA(Y161H)–MnPP]3 cluster. The difference absorption spectrum of Hb–[HSA(Y161H)–MnPP]3 minus Hb–HSA(Y161H)3 (λmax: 560, 593 nm) coincided well with a three-fold-enlarged HSA(Y161H)–MnPP spectrum (Fig. 3). This observation clearly indicates that MnPP is bound within each subdomain IB of HSA(Y161H) unit by an axial His-161 coordination. In marked contrast, attempts to include MnPP into the exterior albumin units of Hb–HSA(wt)3 clusters failed. A possible explanation is that the association of MnPP with HSA might be somewhat sterically hindered in the cluster. The Cys-34 of HSA locates in subdomain IA (Fig. 2A). Therefore, the entrance of the hydrophobic pocket for MnPP in the subdomain IB turns in the direction of the Hb centre. We inferred that a Tyr-161 coordination might be too weak to allow binding of MnPP to the HSA(wt) shell.
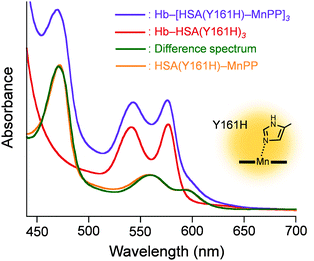 | ||
| Fig. 3 Vis absorption different spectrum of Hb–[HSA(Y161H)–MnPP]3 minus Hb–HSA(Y161H)3, and comparison with that of HSA(Y161H)–MnPP. In PBS (pH 7.4) solution at 25 °C. | ||
The isoelectric point of the Hb–HSA(Y161H)3 cluster was satisfactorily low (pI = 5.1), which is attributed to the negative surface net charges of the exterior HSAs. The pI value of Hb–[HSA(Y161H)–MnPP]3 cluster was also 5.1, implying that MnPP binding caused no alteration of the surface charge of the cluster. Probably, the intravenous transfusion of this hybrid haemoprotein is expected to enable long-term circulation without extravasation because of the electrostatic repulsion between the negatively charged HSA surface and the glomerular basement membrane around the endothelial cells.
The visible absorption spectral maxima based on the Hb component of Hb–[HSA(Y161H)–MnPP]3 in PBS solution were fundamentally the same as those of Hb–HSA(wt)3 and native Hb under N2, O2, and CO atmosphere (deoxy, oxy, and carbonyl forms) (Table S1†).14,24 To evaluate the influence of MnPP on the O2 affinity, the equilibrium between O2 and the Hb–[HSA(Y161H)–MnPP]3 cluster was measured. The P50 value (O2-partial pressure where Hb is half-saturated with O2) and the co-operativity coefficient (Hill coefficient, n) were ascertained to be 9 Torr and 1.5, respectively (Fig. S2, Table S2†). They were identical to the values of Hb–HSA(wt)3.14 Based on these findings, we inferred that the electronic states of the haems in Hb core were unaffected by the association of MnPP to the HSA(Y161H) shell.
Finally, the O2-complex stability of Hb–[HSA(Y161H)–MnPP]3 cluster in aqueous H2O2 solution was investigated. The H2O2 concentration in the normal human plasma is 4–5 μM.25 It is assumed to be tens of micromolars (≤35 μM) under inflammatory conditions.26 Therefore, we examined the oxidation rates of Hb–[HSA(Y161H)–MnPP]3, Hb–HSA(wt)3, and Hb in aqueous 10 μM H2O2 solution. The time courses of the absorbance increase at 630 nm (which results from metHb formation) were markedly different in these protein solutions (Fig. 4). Native Hb showed a biphasic autoxidation curve. Approximately 25% Hb is oxidized in the initial phase within 30 min, followed by a second slow oxidation process. The metHb formation level reached 35% after 120 min. The α subunits in Hb are known to be oxidized more easily than the β subunits.11 Because the haem concentration was 40 μM ([Hb] = 10 μM), oxidation of the 50% α subunit occurred first. The remaining subunits were subsequently oxidized.
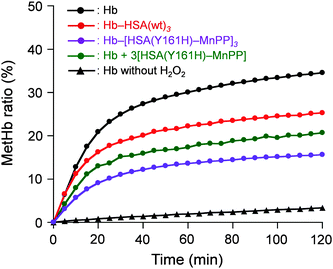 | ||
| Fig. 4 MetHb formation ratio of Hb–[HSA(Y161H)–MnPP]3 cluster in PBS (pH 7.4) solution containing 10 μM H2O2 at 25 °C. | ||
The rate of metHb formation was low in the Hb–HSA(wt)3 cluster. In the initial phase, the metHb level increased to 17% within 30 min, followed by a slow oxidation reaction. The low velocity is likely to result from a wrapping effect of HSA.27 As expected, the Hb–[HSA(Y161H)–MnPP]3 cluster was remarkably stable in the H2O2 solution. We observed only 15% metHb after 120 min, which is 43% of the value of native Hb. This stable O2-complex derives from the catalase activity of the HSA(Y161H)–MnPP units in the periphery. Actually, the oxidation rate of Hb in the coexistence of three-equivalent moles of HSA(Y161H)–MnPP, which are not covalently linked, was higher than that of the cluster. We therefore conclude that the HSA(Y161H)–MnPP shell acts as an efficient scavenger for external H2O2 and thereby achieves protection of the Hb centre.
This report is the first to describe the protein cluster comprising an Hb wrapped by genetically engineered HSA mutants containing MnPP. The HSA(wt)–MnPP complex has weak catalase activity, whereas a site-specific mutation in the subdomain IB of HSA, Tyr-161 → His, confers a five-fold-higher enzyme activity on the prosthetic MnPP group. The Hb–HSA(Y161H)3 cluster can also accommodate MnPP into the external albumin units by axial His-161 coordination. Results show that the Hb–[HSA(Y161H)–MnPP]3 cluster formed an extremely stable O2-complex even in H2O2 solution. This unique super-structured haemoprotein with (i) negative surface net charges, (ii) high O2-affinity, and (iii) catalase activity can be of great importance as an RBC alternative in various medical applications involving O2 delivery to ischaemia-reperfusion tissues in which overproduction of O2˙− and H2O2 occurs.
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Area “Coordination Programming” (Area 2107, No. 21108013) from MEXT Japan, and a Joint Research Grant from the Institute of Science and Engineering, Chuo University.
Notes and references
- J. E. Squires, Science, 2002, 295, 1002–1005 CrossRef CAS PubMed.
- R. M. Winslow, J. Intern. Med., 2003, 253, 508–517 CrossRef CAS.
- J. S. Jahr, A. Sadighi, L. Doherty, A. Li and H. W. Kim, in Chemistry and Biochemistry of Oxygen Therapeutics, ed. A. Mozzarelli and S. Bettati, John Wiley & Sons, Ltd., West Sussex, 2011, pp. 301–316 Search PubMed.
- Hemoglobin-Based Oxygen Carriers as Red Cell Substitutes and Oxygen Therapeutics, ed. H. W. Kim and A. G. Greenburg, Springer-Verlag, Berlin, 2013 Search PubMed.
- S. R. Snyder, E. V. Welty, R. Y. Walder, L. A. Williams and J. A. Walder, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 7280–7284 CrossRef CAS.
- R. Kluger, J. S. Foot and A. A. Vandersteen, Chem. Commun., 2010, 46, 1194–1202 RSC.
- V. T. Rentko, L. B. Pearce, P. F. Moon-Massat and M. S. Gawryl, in Blood Substitutes, ed. R. M. Winslow, Elsevier, San Diego, 2006, pp. 424–436 Search PubMed.
- S. A. Gould and G. S. Moss, World J. Surg., 1996, 20, 1200–1207 CrossRef CAS.
- K. D. Vandegriff, A. Malavalli, J. Wooldridge, J. Lohman and R. M. Winslow, Transfusion, 2003, 43, 509–516 CrossRef CAS.
- T. Hu, D. Li, B. N. Manjula and S. A. Acharya, Biochemistry, 2008, 47, 10981–10990 CrossRef CAS PubMed.
- M. Tsuruga, M. Matsuoka, A. Hachimori, Y. Sugawara and K. Shikama, J. Biol. Chem., 1998, 273, 8607–8615 CrossRef CAS PubMed.
- A. Alagic, A. Koprianiuk and R. Kluger, J. Am. Chem. Soc., 2005, 127, 8036–8043 CrossRef CAS PubMed.
- F. D'Agnilloo and T. M. S. Chang, Nat. Biotechnol., 1998, 16, 667–671 CrossRef PubMed.
- D. Tomita, T. Kimura, H. Hosaka, Y. Daijima, R. Haruki, K. Ludwig, C. Böttecher and T. Komatsu, Biomacromolecules, 2013, 14, 1816–1825 CrossRef CAS PubMed.
- B. J. Day, I. Fridovich and J. D. Crapo, Arch. Biochem. Biophys., 1997, 347, 256–262 CrossRef CAS PubMed.
- S. Curry, H. Madelkow, P. Brick and N. Franks, Nat. Struct. Biol., 1998, 5, 827–835 CrossRef CAS PubMed.
- J. Ghuman, P. A. Zunszain, I. Petitpas, A. A. Bhattacharya, M. Otagiri and S. Curry, J. Mol. Biol., 2005, 353, 38–52 CrossRef CAS PubMed.
- A. A. Bhattacharya, S. Curry and N. Frank, J. Biol. Chem., 2000, 275, 38731–38738 CrossRef CAS PubMed.
- J. Ghuman, P. A. Zunszain, T. Komatsu, E. Tsuchida and S. Curry, BMC Struct. Biol., 2003, 3, 6 CrossRef PubMed.
- T. Komatsu, N. Ohmichi, A. Nakagawa, P. A. Zunszain, S. Curry and E. Tsuchida, J. Am. Chem. Soc., 2005, 127, 15933–15942 CrossRef CAS PubMed.
- R. Kato, K. Kobayashi, M. Akiyama and T. Komatsu, Dalton Trans., 2014, 9, 83–86 CAS.
- T. Yonetani and T. Asakura, J. Biol. Chem., 1969, 244, 4580–4588 CAS.
- Y.-B. Cai, X.-H. Li, J. Jing and J.-L. Zhang, Metallomics, 2013, 5, 828–835 RSC.
- E. Antonini and M. Brunori, Hemoglobin and myoglobin in their reactions with ligands, North-Holland Publisher Co., Amsterdam, p. 19 Search PubMed.
- Y. Yamamoto, M. H. Brodsky, J. C. Baker and B. N. Ames, Anal. Biochem., 1987, 160, 7–13 CrossRef CAS.
- B. Halliwell, M. V. Clement and L. H. Long, FEBS Lett., 2000, 486, 10–13 CrossRef CAS.
- Y. Iwao, Y. Ishima, J. Yamada, T. Noguchi, U. Kragh-Hansen, K. Mera, D. Honda, A. Suenaga and M. Otagiri, IUBMB Life, 2011, 64, 450–454 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, SEC curve, OEC curve, UV-Vis absorption maxima and O2-binding parameters of the Hb–[HSA(Y161H)–MnPP]3 cluster. See DOI: 10.1039/c4cc06076h |
| This journal is © The Royal Society of Chemistry 2014 |
