Ferrocene-based supramolecular structures and their applications in electrochemical responsive systems
Liao
Peng
,
Anchao
Feng
,
Meng
Huo
and
Jinying
Yuan
*
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: yuanjy@mail.tsinghua.edu.cn; Fax: +86-10-62771149; Tel: +86-10-62783668
First published on 14th July 2014
Abstract
Electrochemical stimuli have attracted much attention in recent years as they are simple, clean and can be widely applied in biological systems and material science. As one type of common guest molecules, ferrocene and its derivatives have been well studied with different host molecules, mainly including cyclodextrins, cucurbiturils, pillararenes and calixarenes. This article generally summarizes the recent work on the host–guest interactions between ferrocene derivatives and their host molecules, as well as various supramolecular systems based on these interactions. In addition, the development and outlook of electrochemical responsive systems are also discussed.
1. Introduction
Interest in stimuli-responsive systems, which can undergo reversible physical or chemical changes as a response to external stimuli, has grown tremendously over the past several years. The stimuli range from light,1–6 electrochemistry,7–25 CO226–28 and redox11,29–37 to pH,38–43 temperature44–48 and so on. These stimuli-responsive systems can be widely applied as biological materials and nano machines,9,10,20,21,32,33,49–54 thus drawing more and more attention.As one type of novel stimuli, electrochemical stimuli can drive the structural changes of the systems through inducing electron transfer reactions.55,56 Electrochemical stimuli are considered significantly attractive because their mode and magnitude can be readily regulated using a potentiostat. Also, the electron transfer reaction is well understood51 and the change of potential plays a fundamental role in membrane activities of living organisms, such as membrane fusion and disassembly. Most importantly, electrochemical stimuli are clean and can be realized without adding any extra redox reagents or solutions, which is of significance for their application in biological and material fields, because the accumulation of added substances may affect the viability of cells or the mechanical properties of the materials.
To date, various electrochemical responsive systems have been developed, including systems based on conducting polymers,56 polyelectrolytes23,57,58 and small electroactive molecules, such as tetrathiafulvalene,7 metallocenes8–21 and viologen.14,15,22,59 In this review, we will discuss the most significant progress in supramolecular systems based on ferrocene (Fc) derivatives.
2. Supramolecular systems based on ferrocene derivative guests
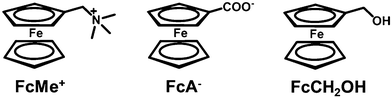
For decades, supramolecular systems have been widely studied due to their dynamic and reversible properties as well as their resulting ability to mimic organisms and to respond to external stimuli. In supramolecular systems based on electroactive guests, the application of a certain magnitude of voltage or current can induce the redox reaction of the guest molecules, thus changing the host–guest interaction in the system. Therefore, we can control the structure of these supramolecular systems by electrochemical method.Fc is widely investigated in electrochemical responsive supramolecular systems because Fc derivatives exhibit simple electrochemical behaviour, and their electroactivity makes it possible to use cyclic voltammetric (CV) techniques to characterize the host–guest interactions. Many types of Fc derivatives, such as ferrocene carboxylic acid (FcA) and ferrocenylmethanol (FcCH2OH) are commercially available or can be readily synthesized. The variation of host molecules also contributes to the progress of the related fields, including cyclodextrins (CD), cucurbiturils (CB), calixarenes (CA) and pillararenes (PA). Different features and mechanisms of these host–guest interactions as well as their applications in constructing supramolecular systems will be discussed below.
2.1 Cyclodextrin (CD)
CD is a macrocyclic oligosugar composed of a certain number of glucoside units with a certain size of non-polar cavity. Many CDs are commercially available, and the hydroxyl groups offer them the opportunity to be easily modified. The most studied CDs are CDs with 6, 7 and 8 glucoside units, namely α-, β- and γ-CD, respectively. CDs can interact with many guests with suitable sizes, such as polyethylene glycol (PEG) chain,60–62 azobenzene,6 adamantine63 and pyrene64 derivatives. Therefore, different supramolecular systems including vesicles, polyrotaxane,60–62 micelles63 and hydrogels6,64 can be constructed based on them.65 When interacting with Fc derivatives, CDs in different sizes form inclusion complexes with different structures (Fig. 1).66–69 Particularly, β-CD has the strongest binding ability under a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of β-CD and Fc, which is widely investigated.
1 molar ratio of β-CD and Fc, which is widely investigated.
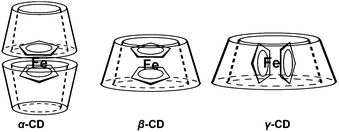 | ||
| Fig. 1 Structures of the inclusion complexes formed from Fc with different sizes of CDs (reproduced from ref. 69). | ||
The basic principle and electrochemical properties of the host–guest interactions between β-CD and Fc were first investigated by Evans et al. in 1985.70 Characterization by circular dichroism and ultraviolet-visible absorption spectroscopy demonstrated that β-CD can form a stable inclusion complex with Fc derivatives in the reduction state, with a formation constant of 2200 M−1 and a configuration where the Cp–Fe–Cp axis is parallel to the axis of the β-CD cavity. Whereas, no binding of β-CD with oxidized Fc can be detected. Additionally, the mechanism of the oxidation of the β-CD/Fc complex was also investigated in this study. The CV curves revealed that the complex is oxidized in a CE (chemical-electrical) process where the complex first dissociates into Fc and β-CD (chemical process), followed by the oxidation of free Fc (electrical process). Hence, no direct oxidation of the complex can occur, which is distinct from other hosts that we will discuss later.
Different from Fc, cobaltocenium (Cob) derivatives in their most stable oxidation state (Cob+) do not interact with β-CD, while their neutral state achieved from one electron reduction is an excellent guest for β-CD.71 Therefore, the neutral state of a metallocene with appropriate size can be strongly bound by β-CD, while the charged state of similar size is not. This is the basis why we can electrochemically control the binding affinity between β-CD and Fc or other metallocene guests by electrochemical oxidation or reduction.
Due to the reversible properties of β-CD/Fc linkage, it has been applied in constructing various supramolecular systems with electrochemical responsiveness, as discussed in the following section.
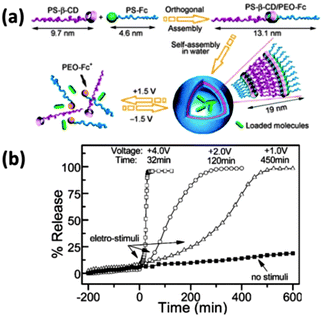 | ||
| Fig. 2 (a) Schematic illustration of the voltage-responsive controlled assembly and disassembly of PS-β-CD/PEO-Fc supramolecular vesicles (potential was reported vs. a saturated Ag/AgCl reference electrode); (b) controlled release of rhodamine B from the supramolecular vesicles upon various voltage stimuli and in comparison with the free release from the vesicles without stimuli (reproduced from ref. 8). | ||
Therefore, the assembly and disassembly of the vesicles can be reversibly manipulated by voltage as an electrochemical method. Additionally, this system can act as an effective carrier in voltage controlled molecule release with a release rate regulated by the strength of the voltage: when the voltage increases from +1.0 to +4.0 V, the time for complete release of rhodamine B decreases from 450 to 32 min (Fig. 2b). This work proved that the electrochemical method can be applied in supramolecular assemblies as a clean type of stimuli and opened up a way for the β-CD/Fc linkage to be applied in constructing nanomaterials.
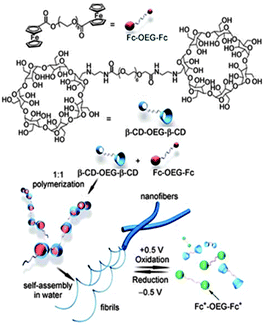 | ||
| Fig. 3 Schematic illustration of the formation of the supramolecular polymer β-CD-OEG-β-CD-Fc-OEG-Fc by β-CD-OEG-β-CD and Fc-OEG-Fc and their fabrication into responsive supramolecular nanofibers with redox-switchable properties. (Potential was reported vs. a saturated Ag/AgCl reference electrode) (reproduced from ref. 69). | ||
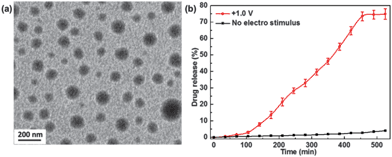 | ||
| Fig. 4 (a) TEM characterization of PEG-β-CD-Fc-PLLA micelles and (b) the dependence on the amount of paclitaxel on time under no stimuli and +1.0 V stimuli. (Potential was reported vs. saturated calomel reference electrode) (reproduced from ref. 10). | ||
Also, we can apply the β-CD/Fc connection as a novel way to get more morphologies of assembly. As an example, micelles with a new type of brush-like supramolecular copolymer topology were recently reported by our group. We synthesized PEG-block-poly(glycidyl methacrylate), decorated with amounts of β-CD pendants (GA-CD) and poly(caprolactone) containing Fc end-capping (L-Fc). Based on the host–guest interactions, they can likewise form a brush-like supramolecular copolymer. The copolymer can further self-assemble in an aqueous solution into micelles with voltage-triggered reversible disassemble and reassemble ability (Fig. 5).11
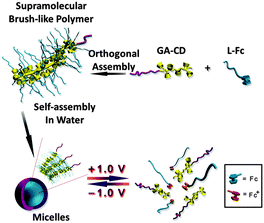 | ||
| Fig. 5 Schematic illustration of the formation of electrochemical redox responsive micelles from brush-like supramolecular block copolymers and their controlled assembly and disassembly. (Potential was reported vs. saturated Ag/AgCl reference electrode) (reproduced from ref. 11). | ||
The first polymeric hydrogel based on the inclusion complex between β-CD and Fc with electrochemical activity and dual responsiveness was reported by Jiang et al.12β-CD and Fc were decorated onto quantum dots and block copolymer to obtain β-CD @ quantum dots (β-CD@QDs) and Fc-poly(N,N-dimethylacrylamide-block-N-isopropyl acrylamide) [Fc-P(DMA-b-NIPAM)], respectively. With β-CD@QDs as the core and Fc-P(DMA-b-NIPAM) as the shell, a hybrid supramolecular polymer Fc-hybrid inclusion complex (Fc-HIC) as well as hydrogel were formed, which are thermo reversible and electrochemically active at elevated temperatures (Fig. 6).
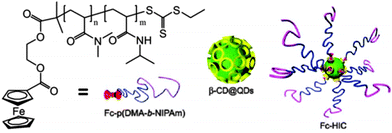 | ||
| Fig. 6 Chemical structure of Fc-P(DMA-b-NIPAM) and schematic illustration of the formation of the supramolecular polymer Fc-HIC by Fc-P(DMA-b-NIPAM) and β-CD@QDs (reproduced from ref. 12). | ||
Harada et al. described another type of hydrogel composed of two polyacrylic acid (PAA)-based polymers and further studied its self-healing ability as a soft material.13 They synthesized β-CD with functionalized PAA (PAA-6-β-CD) and Fc with functionalized PAA (PAA-Fc). Supramolecular hydrogel can form based on the host–guest interaction (Fig. 7a), and the sol–gel transition of the hydrogel can be induced by voltage stimuli as well as redox stimuli (Fig. 7b). Moreover, due to the dynamic binding ability of the β-CD/Fc connection, this hydrogel also has excellent self-healing and self-repairing ability, which can be regulated by redox stimuli. This work demonstrated that the electrochemical method not only can switch the microscale structure of supramolecular complexes based on β-CD and Fc, but also change their macroscale morphology. Therefore, in addition to the soft nature and universal application of hydrogel, more functions of these systems are expected, such as stimuli-responsive media for cell culture, vascular embolization materials and injectable drug carriers in biological field.
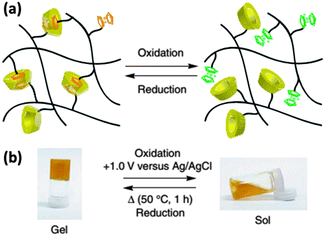 | ||
| Fig. 7 (a) Schematic illustration of sol–gel transition and (b) sol–gel transition experiment using electrochemical reactions of PAA-based hydrogel. (Potential was reported vs. a saturated Ag/AgCl reference electrode) (reproduced from ref. 13). | ||
Apart from the above supramolecular systems constructed on the basis of the host–guest interaction, the supramolecular structure containing only host molecules can be applied to grasp and reversibly release Fc guests. To achieve this, we modified β-CD onto biocompatible comb-copolymer ethyl cellulose-graft-poly(e-caprolactone) (EC-g-PCL) to get EC-g-PCL-β-CD, which can form spherical micelles covered with β-CD receptors on the outside surface (Fig. 8).14 After forming the inclusion complex with FcA, the micelles can reversibly release and grasp FcA under electrochemical stimuli. The study indicated that this self-assembled structure can be a promising vehicle for the delivery and controlled release of various molecules as long as the micelles are modified with Fc moieties.
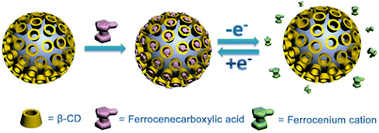 | ||
| Fig. 8 Schematic illustration of the micelles formed from EC-g-PCL-β-CD and its reversible release of FcA (reproduced from ref. 14). | ||
2.2 Cucurbituril (CB)
Cucurbit[n]urils, short as CBns, are synthesized macrocyclic host molecules composed of n glycoluril monomers with a certain size of enclosed cavities.72 As one of the common type of CBns, CB7 has an internal cavity volume of 279 Å3, which is the closest to the 262 Å3 of β-CD.15 While β-CD does not interact with Fc or Cob in their charged state but do in their neutral state as stated before, CB7 can interact with Fc in its neutral state or with cationic groups but cannot bind Fc with anionic groups.The formation of the complex between Fc and CB7, as well as its comparison with β-CD was first investigated by Kaifer et al.16 For neutral and cationic Fc derivatives with suitable size (such as FcCH2OH and FcMe+ in Fig. 9), their binding constants with CB7 are in the range of 109–1010 M−1 and 1012–1015 M−1, respectively,15 which are much greater than 103 M−1, the constants of the same neutral molecules with β-CD. This is because the regions around the carbonyl oxygens in CBs are significantly negative, resulting in strong affinity with positively charged guest molecules. Thus, the strong binding affinity of CB7 with Fc is the combined result of the steric suitability and hydrophobic interactions, as well as the ion–dipole interactions of the two rims and the inner cavity.73 The highly negative regions around the two cavity entrances also leads to the rejection of anionic guests, which can account for the phenomenon that the negatively charged FcA− was not bound by CB7 at all.
Applying the high stability of the CB7-Fc linkage, supramolecular velcro for reversible underwater adhesion was constructed by Kim et al.34 They synthesized a “loop” silicon surface modified with CB7 hosts and a “hook” silicon surface with Fc guests, which can adhere in water to form supramolecular velcro (Fig. 10a). Considering the redox activity of Fc, the supramolecular velcro can be fastened or unfastened by adding ascorbic acid or NaClO to the solution (Fig. 10b and c). If this is the case, we can propose that an electrical field may offer a more efficient alternative to a chemical redox agent for switchable underwater adhesion. Further study revealed the different mechanism for the electron transfer reaction of the complex between β-CD and CB7, as shown in Fig. 11. As stated before, the β-CD-Fc complex is oxidized in a CE scheme where the dissociation occurs in advance of the oxidation (Fig. 11a). This process is typical for the oxidation of the inclusion complex at fast scan rate, because the complex cannot dissociate fast enough to produce enough free guests to be oxidized, thus the resulting CV curves are distorted.70,74 However, the CV curves of the CB7-Fc+ complex remained fully reversible at scan rates as fast as 2 V s−1.73 This means that the electron transfer reaction for the oxidation of Fc uncharacteristically proceeded in its encapsulated form (Fig. 11b).
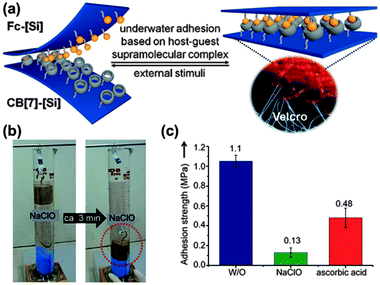 | ||
| Fig. 10 (a) Schematic illustration of supramolecular velcro for underwater adhesion based on the surface modified with CB7 and Fc moiety, respectively. (b) The detachability of supramolecular velcro with an oxidizing reagent (NaClO, 10 mm) and (c) the reversibility of supramolecular velcro by chemical means (W/O = without any additional reagents) (reproduced from ref. 34). | ||
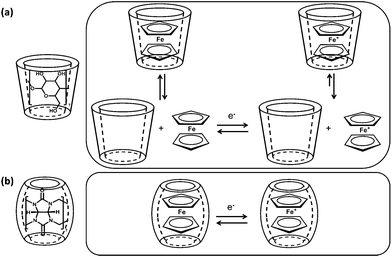 | ||
| Fig. 11 Different mechanisms of the electron transfer reaction of Fc in the presence of β-CD and CB7, respectively. | ||
Additionally, the crystal study reveals significant difference in the structure of the complex between β-CD and CB7. For β-CD-Fc inclusion complex, the axes of Cp–Fe–Cp and β-CD cavity are parallel and it is the only configuration. However, two crystallographically independent structures are found to coexist in the CB7-Fc crystal, which differ on the ferrocene orientation inside the host cavity (Fig. 12).16 In both complexes, the Cp rings of Fc are nearly eclipsed and the Fe atom is located close to the geometric center of the CB7 molecule. The most important difference lies on the angles between the axis of Cp–Fe–Cp and CB7 cavity, which are 22° and 79°, respectively. This crystal study reveals that the Fc enjoys a certain degree of rotational freedom inside the cavity of the CB7 host. This freedom means that any modification or substituents covalently attached to Fc may influence the host guest interactions between the CB7 and Fc guests. For example, attachment to the Fc unit with a single oligoethyleneglycol chain or two substituents as ‘bi-armed’ guests somewhat decreases the binding constants, but the constants remain high and exceed 107 M−1 in all cases.15,75
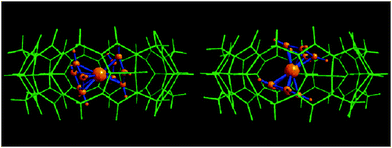 | ||
| Fig. 12 X-Ray crystal structure of the complex between Fc and CB[7] (reproduced from ref. 16). | ||
Another difference between CB7 and β-CD is that the high stability of the inclusion complex still remained after the oxidation between CB7 and Fc guests.15,17 Even after the oxidation and accompanied decrease of the association constant, the CB7-FcMe+ is still extraordinarily stable. This means that the oxidation may not drive enough structural changes, which makes research of the electrochemical control of the complex between the CB7 and Fc guest difficult.
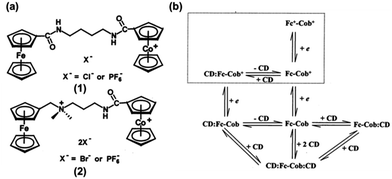
However, Kaifer et al. realized this control through special molecular design. The synthesized guests, Fc2X2+ and Fc2H2+, were composed of two terminal ferrocenylmethylammonium groups linked by different groups (Fig. 13a).18 Upon the addition of 1.0 equiv. of CB7, a stable inclusion complex can form with the CB7 on the terminal Fc residues. However, after one-electron oxidation of both ferrocenes, the host molecule moved to the central binding site on the linker and this change was reversible (Fig. 13b). Therefore, this complex can be seen as stable “pseudorotaxane,” where the position of the host molecules can be accurately and reversibly controlled by electrochemical methods.
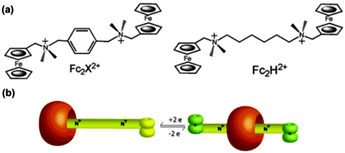 | ||
| Fig. 13 (a) Dinuclear Fc guests for binding to the CB7 host and (b) schematic illustration of the electrochemical control on the CB7 binding location in the pseudorotaxanes formed from CB7 and Fc2X2+ as well as Fc2H2+ (reproduced from ref. 18). | ||
Apart from this work, in most studies of electrochemical control of the complex with CB7, the guests are 4,4′-bipyridine and its derivatives, such as viologen,15,17,22 which we will not discuss here due to the limited space.
2.3 Calixarene (CA)
Calix[n]arenes are synthesized macrocyclic host molecules based on the hydroxyalkylation products of phenols and aldehydes. The hydrophobic internal cavities of the CAs vary by the number of repeated units, and the solubility of the molecules can also be readily adjusted by different modification.76 Different from CD and CB, CA has a flexible structure and usually a poorly defined cavity, so modification and the environment can greatly affect the host–guest interactions. On one hand, modification should be cautious and variation in temperature, pH and solvent can significantly affect the study of CA. On the other hand, this means that the host–guest interactions are sensitive and can respond to more types of stimuli. Another difference is that the CA itself can be electro- and redox-active by attaching electroactive substituents or converting the phenolic moieties in the ring to quinone,77–79 which endows more opportunities as well as challenges for the electrochemical control of Fc guest bound by CAs.The first research of Fc derivatives with sulfonated C[6]A (SC[6]A, Fig. 14a and b) was conducted by Kaifer et al.20 Electrochemical and 1H NMR results showed that SC[6]A can bind not only to neutral FcCH2OH, but also to positively charged FcMe+. However, SC[6]A forms a stronger complex with FcMe+ than FcCH2OH, also stronger with the oxidized form Fc+ than the reduced form Fc. Through the calculation process the dependence of the apparent diffusion coefficient on CA concentration, the equilibrium binding constants between SC[6]A and FcMe+ as well as FcCH2OH are 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 ± 960 dm3 M−1 and 3650 ± 360 dm3 M−1, respectively. The large constant for neutral FcCH2OH indicates the primary significance of the non-electrostatic interactions, while the distinction between these two guests demonstrates that the electrostatic interactions between FcMe+ and the negative charge of the sulfonate ions also plays a secondary role in the binding process.
930 ± 960 dm3 M−1 and 3650 ± 360 dm3 M−1, respectively. The large constant for neutral FcCH2OH indicates the primary significance of the non-electrostatic interactions, while the distinction between these two guests demonstrates that the electrostatic interactions between FcMe+ and the negative charge of the sulfonate ions also plays a secondary role in the binding process.
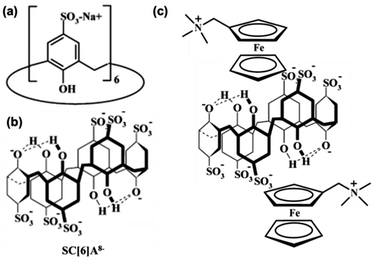 | ||
Fig. 14 (a), (b) Structures of SC[6]A and (c) its 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 inclusion complex formed with FcMe+ (reproduced from ref. 78). 2 inclusion complex formed with FcMe+ (reproduced from ref. 78). | ||
Further study gives more details about the host–guest interactions between SC[6]A and Fc derivatives.78 When SC[6]A forms a stable complex with FcMe+ in a neutral aqueous solution at room temperature, the SC[6]A is in its 1,2,3-alternate conformation and the molar ratio of FcMe+ to SC[6]A is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 15c), which is different from the inclusion complex of β-CD and CB7. In this rather rigid conformation, one SC[6]A molecule presents two identical cavities, each with four negative charges, and they are only suitable for two FcMe+ guest molecules.
1 (Fig. 15c), which is different from the inclusion complex of β-CD and CB7. In this rather rigid conformation, one SC[6]A molecule presents two identical cavities, each with four negative charges, and they are only suitable for two FcMe+ guest molecules.
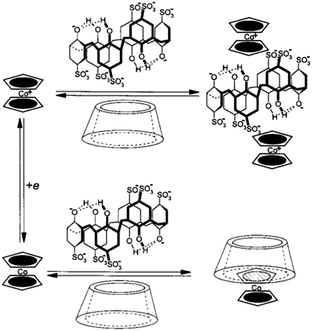 | ||
| Fig. 15 Selective binding of the oxidized and reduced forms of Cob guest with SC[6]A and β-CD under the control of electrochemical stimuli (reproduced from ref. 81). | ||
Moreover, the influences of solvent, pH and temperature on the conformation as well as the host–guest interactions were studied. The change to dimethyl sulfoxide as solvent can stabilize the inclusion complex because it can lock the SC[6]A in its 1,2,3-alternate conformation, while a decrease in pH from 7.0 to 2.6 or rise in temperature decreases the stability of the complex due to the loss of the 1,2,3-alternate conformation. In addition, other study emphasizes the significance of intermolecular hydrogen bonding in the lower rim of the calixarene in the host–guest interactions,80 which is in accordance with the above results.
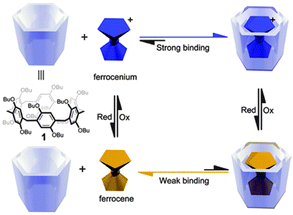
Considering the distinct binding affinity of Fc or Cob derivatives with CA and β-CD, selective binding of the Cob guest was achieved based on the different electrochemical condition.81 In the mixed solution of SC[6]A and β-CD, Cob+ forms a stable 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with SC[6]A, while it does not interact with β-CD. However, after one-electron reduction of Cob+ to Cob, the binding affinity is reversed. That is, Cob forms a stable 1
1 complex with SC[6]A, while it does not interact with β-CD. However, after one-electron reduction of Cob+ to Cob, the binding affinity is reversed. That is, Cob forms a stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex with β-CD but does not bind with SC[6]A (Fig. 15). Though Cob was used here as a representative of a metallone guest because of the stability of the inclusion complex, FcA also has the potential to conduct a similar function. This work presents us a simple example as to how the electrochemical control can be manipulated to realize the selective binding with different hosts.
1 inclusion complex with β-CD but does not bind with SC[6]A (Fig. 15). Though Cob was used here as a representative of a metallone guest because of the stability of the inclusion complex, FcA also has the potential to conduct a similar function. This work presents us a simple example as to how the electrochemical control can be manipulated to realize the selective binding with different hosts.
2.4 Pillararene (PA)
Pillar[n]arenes are macrocyclic host molecules composed of a certain number of hydroquinone units linked by methylene bridges at the para-positions.82 They have intrinsically symmetrical and rigid pillar structures and can be easily modified to adjust their solubility in different solvents, which brings advantages to their research. However, their disadvantages are that the suitable guests for P[6]As are limited,83–85 and the binding constants are usually less than 104 M−1, except in the research of paraquat, a derivative of 4,4′-bipyridine, by Huang et al.43,86Moreover, Wang et al. first investigated the host guest interactions between P[6]A and Fc as well as Cob in 2013 (Fig. 16).19 When the molar ratio of per-butylated P[6]A (BP[6]A) and Fc is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Fc shows extremely low binding ability with BP[6]A (18 ± 0.5 M−1) in a CDCl3–CD3CN solution. However, the oxidation status, Fc+, can strongly bind with the host molecule, due to the efficient charge transfer from the electron-rich cavity of BP[6]A to Fc. Considering the paramagnetic property of Fc+, Cob+ was used as the analogue of Fc+ to calculate the binding constants, which was about (3.7 ± 1.0) × 104 M−1 in a CDCl3–DMSO-d6 solution. The considerable change of three to four orders of magnitude in the binding ability means that the host–guest interactions between Fc and BP[6]A also has the potential to be applied in electrochemical controlled systems.
1, Fc shows extremely low binding ability with BP[6]A (18 ± 0.5 M−1) in a CDCl3–CD3CN solution. However, the oxidation status, Fc+, can strongly bind with the host molecule, due to the efficient charge transfer from the electron-rich cavity of BP[6]A to Fc. Considering the paramagnetic property of Fc+, Cob+ was used as the analogue of Fc+ to calculate the binding constants, which was about (3.7 ± 1.0) × 104 M−1 in a CDCl3–DMSO-d6 solution. The considerable change of three to four orders of magnitude in the binding ability means that the host–guest interactions between Fc and BP[6]A also has the potential to be applied in electrochemical controlled systems.
 | ||
| Fig. 16 Schematic illustration of the formation of a highly stable redox-responsive inclusion complex based on BP[6]A and Fc (reproduced from ref. 19). | ||
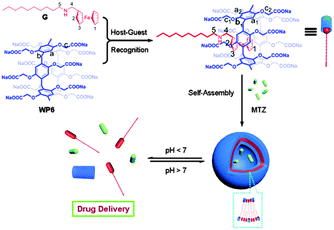
To utilize the host–guest interactions of PA and Fc in stimuli responsive systems, water soluble P[6]A (WP[6]A) with carboxylate groups and Fc derivatives with hydrophobic chains were synthesized by the same group (Fig. 17).42 The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex is stable with a binding constant of (1.27 ± 0.42) × 105 M−1, and it can further assemble into supramolecular vesicles in neutral and alkaline aqueous solutions. As the protonation of the carboxylate group in WP[6]A can be manipulated by a change of pH,87 this supramolecular vesicle is also pH responsive and can be applied in pH controlled drug release. When adjusting the solution pH to 6.0, the hydrophilic COO− changes to insoluble COOH, thus resulting in the disassembly of the vesicles and accompanied drug release. Although the interaction in this research reported to be pH-responsive, it has the potential to be electrochemical responsive through appropriate molecular design.
1 inclusion complex is stable with a binding constant of (1.27 ± 0.42) × 105 M−1, and it can further assemble into supramolecular vesicles in neutral and alkaline aqueous solutions. As the protonation of the carboxylate group in WP[6]A can be manipulated by a change of pH,87 this supramolecular vesicle is also pH responsive and can be applied in pH controlled drug release. When adjusting the solution pH to 6.0, the hydrophilic COO− changes to insoluble COOH, thus resulting in the disassembly of the vesicles and accompanied drug release. Although the interaction in this research reported to be pH-responsive, it has the potential to be electrochemical responsive through appropriate molecular design.
 | ||
| Fig. 17 Schematic illustration of the formation of supramolecular vesicles and their pH-responsive drug release (reproduced from ref. 42). | ||
It is worth mentioning that although the host–guest interactions between CA as well as PA with Fc derivatives are clearly investigated, only a few of them have been introduced to polymer self-assembly or electrochemical supramolecular systems, which means that there is still a wide space for macromolecular scientists to explore.
3. Conclusion and outlook
This article systematically summarizes the recent work on the host–guest interactions between Fc derivatives and various host molecules, namely CD, CB, CA and PA, as well as their applications in constructing various supramolecular systems, especially for electrochemical responsive ones. Different types of host molecules have different interactions with Fc derivatives, which can be summarized in Table 1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8–19,34,42,75–88
8–19,34,42,75–88
| Host molecules | Fc derivatives | Fc+ derivatives | |
|---|---|---|---|
a Solvent is water when not mentioned.
b The results are from SC[6]A.
c Molar ratio of formed inclusion complex is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for FcMe+ and SC[6]A.
d Related data have not been reported. 1 for FcMe+ and SC[6]A.
d Related data have not been reported.
|
|||
| β-CD | Bind neutral, cationic and anionic Fc; the binding constant is about 103–105 M−1 | Does not bind | |
| CB7 | Strongly bind neutral and cationic Fc, does not interact with anionic Fc; the binding constant is about 109–1010 M−1 for neutral Fc and 1012–1015 M−1 for cationic Fc | Weakly binds the oxidized form Fc+ than reduced Fc (approx. 1.5 orders of magnitude in the K value) but retain strong | |
| C[6]A | Bind neutral and cationic Fcc; the binding constant is about 105 M−1 for neutral Fc and 103 M−1 for cationic Fc | Strongly binds the oxidized form Fc+ than reduced Fc | |
| P[6]A | BP[6]A | Weakly bind neutral Fc; the binding constant is about 10 M−1 for neutral Fc in CDCl3–CD3CN | Strongly binds Fc+; the binding constant is about 104 M−1 for Fc+ in a CDCl3–DMSO-d6 solution |
| WP[6]A | Strongly bind Fc; the binding constant is about 105 M−1 for Fc | —d | |
Various host–guest interactions have different advantages that can be applied in various systems. For example, the considerable change of the binding ability between Fc derivatives and β-CD or WP[6]A before and after the oxidation of the Fc moiety endows them great opportunities to be widely utilized in water-soluble electrochemical supramolecular systems. Meanwhile, the system based on hydrophobic P[6]A can be exploited in other solvents, such as CHCl3 or CH3CN. The high stability of the inclusion complexes between CB7 and Fc derivatives makes them used less often in electro-controlled systems, except unique molecular design, but this interaction can be applied to stabilize the active oxidation state of Fc or to be used for strong adhesion, such as supramolecular velcro.34 In addition, the difference in various host–guest interactions gives the possibility of selective binding of the guest in complex systems containing various host molecules,81 or selective binding of the host when containing various guest molecules (Fig. 18).88
 | ||
| Fig. 18 (a) Two heterobimetallic guests examples and (b) electrochemical as well as the chemical equilibria involved in the voltammetric behaviour of the heterobimetallic complex (2) in the presence of the β-CD host (reproduced from ref. 88). | ||
These supramolecular structures with electrochemical responsiveness have tremendous potential with applications in numerous fields. As for different supramolecular structures, electrochemical responsive micelles and vesicles can be used as drug delivery vehicles for voltage-controlled drug release,8,10 while electrochemical responsive hydrogels are able to function as injectable drug carriers, cell culture media and self-healing materials.6,11,13 More applications, such as energy storage, photovoltaic conversion and chemical sensors can also be expected from these systems. However, many problems are still unsolved for these systems. First, the graft of host molecules and Fc derivatives onto polymers largely affects the solubility of the polymers in an aqueous solution. Therefore, a water-soluble polymer backbone is more likely to be chosen, which limits the applications of the systems. Second, the modification and the grafting efficiency of host molecules and Fc onto polymers are not high enough to be applied in industry and needs to be improved. For example, the current modification methods require many steps, and the yield of the most common reaction of β-CD to mono-6-OTs-β-CD is less than 20%.89
As for the applications of current electrochemical stimuli systems, most research has stayed in the primary stage, for example, drug release in vitro. More biological applications have not yet been implemented, perhaps because current experiments for electrochemical stimuli, including high concentration of electrolyte, relatively high potential and the insertion of electrode, may cause damage to cells or tissues. Meanwhile, an electrochemical workstation is necessary for the stimuli experiment, which is not mobile and fast enough to be applied practically. Therefore, further investigation and improvement is needed for practical and real-life applications. Apart from the supramolecular systems based on the interaction between different host molecules and relatively simple Fc derivatives, redox and electrochemical responsive systems based on poly(ferrocenylsilane) (PFS), which contains Fc units in the polymer chain, are also significantly attractive. PFS can be reversibly oxidized and reduced by chemical and electrochemical means, thus can be utilized to construct electroactive materials, such as PFS monolayers on gold,25 composite-wall microcapsules,35PFS polyelectrolyte hydrogels37 and so on.90–92
In conclusion, considering the advantages of Fc derivatives and different types of host molecules, as well as the superiority of the electrochemical method as a clean and novel type of stimulus, supramolecular systems based on Fc derivatives that can respond to electrochemical stimuli will greatly develop in the near future.
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (21174076, 21374053) and Tsinghua University Initiative Scientific Research Program (2012z023998).
Notes and references
- J. L. Mynar, A. P. Goodwin, J. A. Cohen, Y. Z. Ma, G. R. Fleming and J. M. Fréchet, Chem. Commun., 2007, 2081 RSC.
- X. K. Liu and M. Jiang, Angew. Chem., Int. Ed., 2006, 45, 3846 CrossRef CAS PubMed.
- Q. Yan, J. Hu, R. Zhou, Y. Ju, Y. W. Yin and J. Y. Yuan, Chem. Commun., 2012, 48, 1913 RSC.
- H. J. Zhang, Y. Xin, Q. Yan, L. L. Zhou, L. Peng and J. Y. Yuan, Macromol. Rapid Commun., 2012, 33, 1952 CrossRef CAS PubMed.
- L. Cai, J. Lu, V. Sheen and S. F. Wang, Biomacromolecules, 2012, 5, 1663 CrossRef PubMed.
- Y. Takashima, S. Hatanaka, M. Otsubo, M. Nakahata, T. Kakuta, A. Hashidzume, H. Yamaguchi and A. Harada, Nat. Commun., 2012, 3, 1270 CrossRef PubMed.
- M. Asakawa, P. R. Ashton, V. Balzani, A. Credi, C. Hamers, G. Mattersteig, M. Montalti, A. N. Shipway, N. Spencer, J. F. Stoddart, M. S. Tolley, M. Venturi, A. J. P. White and D. J. Williams, Angew. Chem., Int. Ed., 1998, 37, 333 CrossRef CAS.
- Q. Yan, J. Y. Yuan, Z. N. Cai, Y. Xin, Y. Kang and Y. W. Yin, J. Am. Chem. Soc., 2010, 132, 9268 CrossRef CAS PubMed.
- Q. Yan, A. C. Feng, H. J. Zhang, Y. W. Yin and J. Y. Yuan, Polym. Chem., 2013, 4, 1216 RSC.
- L. Peng, A. C. Feng, H. J. Zhang, H. Wang, C. M. Jian, B. W. Liu, W. P. Gao and J. Y. Yuan, Polym. Chem., 2014, 5, 1751 RSC.
- A. C. Feng, Q. Yan, H. J. Zhang, L. Peng and J. Y. Yuan, Chem. Commun., 2014, 50, 4740 RSC.
- P. Du, J. H. Liu, G. S. Chen and M. Jiang, Langmuir, 2011, 27, 9602 CrossRef CAS PubMed.
- M. Nakahata, Y. Takashima, H. Yamaguchi and A. Harada, Nat. Commun., 2011, 2, 511 CrossRef PubMed.
- Y. Kang, J. Y. Yuan, Q. Yan, L. Y. Zheng and L. L. Zhou, Polym. Adv. Technol., 2012, 23, 255 CrossRef CAS.
- S. Gadde, E. K. Batchelor and A. E. Kaifer, Aust. J. Chem., 2010, 63, 184 CrossRef CAS.
- W. S. Jeon, K. Moon, S. H. Park, H. Chun, Y. H. Ko, J. Y. Lee, E. S. Lee, S. Samal, N. Selvapalam, M. V. Rekharsky, V. Sindelar, D. Sobransingh, Y. Inoue, A. E. Kaifer and K. Kim, J. Am. Chem. Soc., 2005, 127, 12984 CrossRef CAS PubMed.
- A. E. Kaifer, W. Li and S. Yi, Isr. J. Chem., 2011, 51, 496 CrossRef CAS.
- D. Sobransingh and A. E. Kaifer, Org. Lett., 2006, 8, 3247 CrossRef CAS PubMed.
- W. Xia, X. Y. Hu, Y. Chen, C. Lin and L. Y. Wang, Chem. Commun., 2013, 49, 5085 RSC.
- L. Zhang, A. Macias, T. Lu, J. I. Gordon, G. W. Gokel and A. E. Kaifer, J. Chem. Soc., Chem. Commun., 1993, 1017 RSC.
- F. Zuo, C. Luo, X. B. Ding, Z. Zheng, X. Cheng and Y. Peng, Supramol. Chem., 2008, 20, 559 CrossRef CAS.
- Q. An, J. Brinkmann, J. Huskens, S. Krabbenborg, J. Boer and P. Jonkheijm, Angew. Chem., Int. Ed., 2012, 51, 12399 CrossRef.
- M. P. Weir, S. Y. Heriot, S. J. Martin, A. J. Parnell, S. A. Holt, J. R. P. Webster and R. A. L. Jones, Langmuir, 2011, 27, 11000 CrossRef CAS PubMed.
- I. Tomatsu, A. Hashidzume and A. Harada, Macromol. Rapid Commun., 2006, 27, 238 CrossRef CAS.
- M. Péter, R. G. H. Lammertink, M. A. Hempenius and G. J. Vancso, Langmuir, 2005, 21, 5115 CrossRef PubMed.
- Q. Yan, R. Zhou, C. K. Fu, H. J. Zhang, Y. W. Yin and J. Y. Yuan, Angew. Chem., Int. Ed., 2011, 50, 4923 CrossRef CAS PubMed.
- D. H. Han, O. Boissiere, S. Kumar, X. Tong, L. Tremblay and Y. Zhao, Macromolecules, 2012, 45, 7440 CrossRef CAS.
- Q. Yan, J. B. Wang, Y. Y. Wu and Y. J. Yuan, Angew. Chem., Int. Ed., 2013, 52, 5070 CrossRef CAS PubMed.
- A. Napoli, M. Valentini, N. Tirelli, M. Müller and J. A. Hubbell, Nat. Mater., 2004, 3, 183 CrossRef CAS PubMed.
- K. N. Power-Billard, R. J. Spontak and I. Manners, Angew. Chem., Int. Ed., 2004, 43, 1260 CrossRef CAS PubMed.
- A. Klaikherd, C. Nagamani and S. Thayumanavan, J. Am. Chem. Soc., 2009, 131, 4830 CrossRef CAS PubMed.
- N. Ma, Y. Li, H. P. Xu, Z. Q. Wang and X. Zhang, J. Am. Chem. Soc., 2010, 132, 442 CrossRef CAS PubMed.
- J. J. Tan, H. L. Kang, R. G. Liu, D. Q. Wang, X. Jin, Q. M. Li and Y. Huang, Polym. Chem., 2011, 2, 672 RSC.
- Y. Ahn, Y. Jang, N. Selvapalam, G. Yun and K. Kim, Angew. Chem., Int. Ed., 2013, 52, 3140 CrossRef CAS PubMed.
- Y. Ma, W. F. Dong, M. A. Hempenius, H. Möhwald and G. J. Vancso, Nat. Mater., 2006, 5, 724 CrossRef CAS PubMed.
- M. Nakahata, Y. Takashima, A. Hashidzume and A. Harada, Angew. Chem., Int. Ed., 2013, 52, 5731 CrossRef CAS PubMed.
- M. A. Hempenius, C. Cirmi, J. Song and G. Julius Vancso, Macromolecules, 2009, 42, 2324 CrossRef CAS.
- E. R. Gillies, T. B. Jonsson and J. M. Fréchet, J. Am. Chem. Soc., 2004, 126, 11936 CrossRef CAS PubMed.
- K. E. B. Doncom, C. F. Hansell, P. Theato and R. K. O'Reilly, Polym. Chem., 2012, 3, 3007 RSC.
- Z. K. Wang, L. H. Wang, J. T. Sun, L. F. Han and C. Y. Hong, Polym. Chem., 2013, 4, 1694 RSC.
- S. Lin, F. S. Du, Y. Wang, S. P. Ji, D. H. Liang, L. Yu and Z. C. Li, Biomacromolecules, 2008, 9, 109 CrossRef CAS PubMed.
- Q. P. Duan, Y. Cao, Y. Li, X. Y. Hu, T. X. Xiao, C. Lin, Y. Pan and L. Y. Wang, J. Am. Chem. Soc., 2013, 135, 10542 CrossRef CAS PubMed.
- X. F. Ji, J. Z. Chen, X. D. Chi and F. H. Huang, ACS Macro Lett., 2014, 3, 110 CrossRef CAS.
- L. Hu and M. J. Serpe, Chem. Commun., 2013, 49, 2649 RSC.
- M. I. Gibson and R. K. O'Reilly, Chem. Soc. Rev., 2013, 42, 7204 RSC.
- Y. Z. You, J. J. Yan, Z. Q. Yu, M. M. Cui, C. Y. Hong and B. J. Qu, J. Mater. Chem., 2009, 19, 7656 RSC.
- Q. Yan, J. Y. Yuan, W. Z. Yuan, M. Zhou, Y. W. Yin and C. Y. Pan, Chem. Commun., 2008, 6188 RSC.
- H. A. Zayas, A. Lu, D. Valade, F. Amir, Z. Jia, R. K. O'Reilly and M. Monterio, ACS Macro Lett., 2013, 2, 327 CrossRef CAS.
- D. Steinhilber, F. Paulus, A. T. Zill, S. C. Zimmerman and R. Haag, MRS Proc., 2012, 1403, 185 Search PubMed.
- M. Robin, A. B. Mabire, J. Damborsky, E. Thom, U. Winzer-Serhan, J. Raymond and R. K. O'Reilly, J. Am. Chem. Soc., 2013, 135, 9518 CrossRef CAS PubMed.
- A. E. Kaifer, Acc. Chem. Res., 1999, 32, 62 CrossRef CAS.
- J. J. Schmidt, J. H. Jeong, R. Kohman, A. Zill, R. DeVolder, S. C. Zimmerman and H. Kong, J. Am. Chem. Soc., 2013, 135, 8770 CrossRef PubMed.
- A. Zill, A. Rutz, R. E. Kohman, A. Alkilany, C. J. Murphy, H. Kong and S. C. Zimmerman, Chem. Commun., 2011, 47, 1279 RSC.
- M. R. Islam and M. J. Serpe, Chem. Commun., 2013, 49, 2646 RSC.
- S.-k. Ahn, R. M. Kasi, S.-C. Kim, N. Sharma and Y. X. Zhou, Soft Matter, 2008, 4, 1151 RSC.
- R. Shankar, T. K. Ghosh and R. J. Spontak, Soft Matter, 2007, 3, 1116 RSC.
- J. Shang, Z. Z. Shao and X. Chen, Biomacromolecules, 2008, 9, 1208 CrossRef CAS PubMed.
- M. T. Popescu, I. Athanasoulias, C. Tsitsilianis, N. A. Hadjiantoniou and C. S. Patrickios, Soft Matter, 2010, 6, 5417 RSC.
- A. Mirzoian and A. E. Kaifer, Chem. – Eur. J., 1997, 3, 1052 CrossRef CAS.
- A. Harada, J. Li and M. Kamachi, Nature, 1992, 356, 325 CrossRef CAS.
- Y. L. Wu and J. Li, Angew. Chem., Int. Ed., 2009, 48, 3842 CrossRef CAS PubMed.
- A. Harada, J. Li and M. Kamachi, Nature, 1994, 370, 126 CrossRef CAS.
- J. Wang and M. Jiang, J. Am. Chem. Soc., 2006, 128, 3703 CrossRef CAS PubMed.
- B. Chen, K. L. Liu, Z. X. Zhang, X. P. Ni, S. H. Goh and J. Li, Chem. Commun., 2012, 48, 5638 RSC.
- G. S. Chen and M. Jiang, Chem. Soc. Rev., 2011, 40, 2254 RSC.
- A. Harada and S. Takahashi, J. Chem. Soc., Chem. Commun., 1984, 645 RSC.
- A. Harada, Y. Hu, S. Yamamoto and S. Takahashi, J. Chem. Soc., Dalton Trans., 1988, 729 RSC.
- Y. Odagaki, K. Hirotsu, T. Higuch, A. Harada and S. Takahash, J. Chem. Soc., Perkin Trans. 1, 1990, 1230 RSC.
- A. Harada, Acc. Chem. Res., 2001, 34, 456 CrossRef CAS PubMed.
- T. Matsue, D. H. Evans, T. Osa and N. Kobayashi, J. Am. Chem. Soc., 1985, 107, 3411 CrossRef CAS.
- Y. Wang, S. Mendoza and A. E. Kaifer, Inorg. Chem., 1998, 37, 317 CrossRef CAS.
- J. W. Lee, S. Samal, N. Selvapalam, H. Kim and K. Kim, Acc. Chem. Res., 2003, 36, 621 CrossRef CAS PubMed.
- W. Ong and A. E. Kaifer, Organometallics, 2003, 22, 4181 CrossRef CAS.
- R. Isnin, C. Salam and A. E. Kaifer, J. Org. Chem., 1991, 56, 35 CrossRef CAS.
- L. Cui, S. Gadde, W. Li and A. E. Kaifer, Langmuir, 2009, 25, 13763 CrossRef CAS PubMed.
- T. D. Chung and H. Kim, J. Inclusion Phenom. Mol. Recognit. Chem., 1998, 32, 179 CrossRef CAS.
- A. Pailleret, N. Magan-Oliva, S. Ollivier and D. W. M. Arrigan, J. Electroanal. Chem., 2001, 508, 81 CrossRef CAS.
- J. Alvarez, Y. Wang, W. Ong and A. E. Kaifer, J. Supramol. Chem., 2001, 1, 269 CrossRef CAS.
- M. G. Kaifer, P. A. Reddy, C. D. Cutsche and L. Echegoyen, J. Am. Chem. Soc., 1994, 116, 3580 CrossRef.
- J. Alverez, Y. Wang, M. Gómez-Kaifer and A. E. Kaifer, Chem. Commun., 1998, 1455 RSC.
- Y. Wang, J. Alvarez and A. E. Kaifer, Chem. Commun., 1998, 1457 RSC.
- T. Ogoshi and T. Yamagishi, Eur. J. Org. Chem., 2013, 2961 CrossRef CAS.
- T. Ogoshi, H. Kayama, D. Yamafuji, T. Aoki and T. Yamagishi, Chem. Sci., 2012, 3, 3221 RSC.
- G. Yu, C. Han, Z. Zhang, J. Chen, X. Yan, B. Zheng, S. Liu and F. H. Huang, J. Am. Chem. Soc., 2012, 134, 8711 CrossRef CAS PubMed.
- T. Ogoshi, K. Kida and T. Yamagishi, J. Am. Chem. Soc., 2012, 134, 20146 CrossRef CAS PubMed.
- G. Yu, X. Zhou, Z. Zhang, C. Han, Z. Mao, C. Gao and F. H. Huang, J. Am. Chem. Soc., 2012, 134, 19489 CrossRef CAS PubMed.
- G. C. Yu, M. Xue, Z. B. Zhang, J. Y. Li, C. Y. Han and F. H. Huang, J. Am. Chem. Soc., 2012, 134, 13248 CrossRef CAS PubMed.
- B. González, I. Cuadrado, B. Alonso, C. M. Casado, M. Morán and A. E. Kaifer, Organometallics, 2002, 21, 3544 CrossRef.
- R. C. Petter, J. S. Salek, T. C. Sikorski, G. Kumaravel and F. T. Lin, J. Am. Chem. Soc., 1990, 112, 3860 CrossRef CAS.
- I. Manners, Chem. Commun., 1999, 857 RSC.
- K. Kulbaba and I. Manners, Macromol. Rapid Commun., 2001, 22, 711 CrossRef CAS.
- G. R. Whittell and I. Manners, Adv. Mater., 2007, 19, 3439 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |