Open Access Article
Open Access ArticleSequential Bi–C bond activation reactions of BiEt3via insertion reactions of RE {R = HC[C(Me)N(2,6-i-Pr2C6H3)]2; E = Al, Ga, In}†‡
C.
Ganesamoorthy
,
D.
Bläser
,
C.
Wölper
and
S.
Schulz
*
University of Duisburg-Essen, Universitätsstr. 5-7, S07 S03 C30, D-45117 Essen, Germany. E-mail: stephan.schulz@uni-due.de; Fax: +49-201-183 3830; Tel: +49 201 1834635
First published on 27th August 2014
Abstract
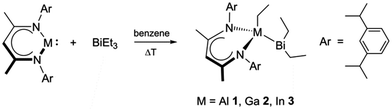
Two of the Bi–C bonds of BiEt3 are sequentially activated by mono-valent RM {R = HC[C(Me)N(2,6-i-Pr2C6H3)]2; M = Al, Ga, In}. The first Bi–C bond activation leads to the formation of insertion complexes, [RMEt(BiEt2)] (M = Al 1; Ga 2; In 3), whereas the consecutive second activation proceeds through a reductive elimination of RMEt2 (M = Al 4, Ga 5), elemental Bi and BiEt3.
Univalent group 13 diyls RM (M = Al, Ga, In; R = halides, Cp*, diketiminates, terphenyl, …) have been found to attract widespread interest from the days of their discovery and spectacular new research fields in modern inorganic chemistry have been developed in the last two decades.1 The fundamental work of Schnöckel et al. led to the isolation of meta-stable MI halides, which smoothed the way to obtain univalent group 13 complexes as well as metalloid Al and Ga clusters by controlling the disproportionation reaction of MI in the presence of suitable organic ligands.2 In contrast, Uhl, Roesky, Jutzi, Power, Robinson and others independently synthesized RM compounds using standard reduction reactions and investigated their diverse reactivity toward a number of unsaturated organic functional groups, such as olefins, diketones, isocyanides, azobenzene, azides and small molecules such as O2, S8, P4, N2O and carbenes.3,4 Moreover, the Fischer group predominantly uses the metal centered lone pair of RM as an “exotic σ-donor” in the formation of late transition metal based molecular compounds and clusters via substitution of weakly bonded ligands.5 The redox behavior of RM was found to play a crucial role in both small molecule activation and in intermetallic compounds/cluster formation reactions. In addition, Lewis acid–base adducts of univalent group 13 metals were explored.6 Our general interest in weak metal–metal bonds7 and Lewis acid–base interactions8 prompted us to focus our attention on the reactions of univalent (RM) and trivalent (R′3E) complexes of group 13 and 15 elements, both of which have different kinds of electron lone pairs. The question was whether these complexes simply behave as donor–acceptor derivatives and, if so, what are the orbital contributions, or do they offer further reactions? We herein report on the reactions of the mono-valent group 13 complexes RM {R = HC[C(Me)N(2,6-i-Pr2C6H3)]2; M = Al, Ga, In} with BiEt3.
RM initially reacts with BiEt3via insertion into one of the Bi–C bonds to yield [RMEt(BiEt2)] (E = Al 1; Ga 2; In 3) (Scheme 1). The yields of 1–3 strongly depend on the reaction temperature and heating time as they normally decompose under harsh conditions. The presence of a slight excess of BiEt3 improves the yield of 3. 1–3 are moisture sensitive but moderately stable in air, decompose at elevated temperatures but are quite stable in solution and in the solid state at ambient temperature under an inert gas atmosphere.
The 1H and 13C NMR spectra of 1–3 show the characteristic resonances of the organic substituents. Due to the presence of two different substituents at M (Et, BiEt2), the resonances due to the i-Pr groups of the β-diketiminate ligand are magnetically inequivalent. The 1H NMR spectra display two septets and four doublets for the two sets of methine and diastereotopic methyl groups of the isopropyl substituents, respectively. In contrast, the γ-H and two methyl groups of the C3N2M ring are in the mirror plane and exhibit only single resonances in the 1H NMR spectra. The 1H and 13C{1H} NMR spectral patterns of 1–3 are comparable to those reported for similar β-diketiminate group 13 complexes.9
The molecular structures of 1–3 were unambiguously confirmed using single crystal X-ray analyses. Suitable crystals of 1–3 were obtained from saturated solutions in n-hexane upon storage at −30 °C after 24 h. Since the conformations in 1–3 are roughly the same, only the molecular structure of 1 is presented (Fig. 1). Crystal data and the details of the structure determination are summarized in the ESI,‡ while selected bond lengths and bond angles are given in Table 1.
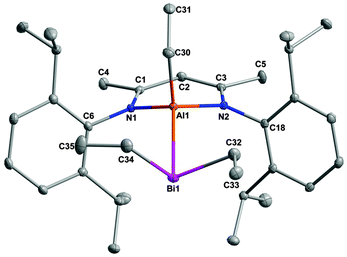 | ||
| Fig. 1 Molecular structure of RAlEt(BiEt2) (1). H-atoms have been omitted for clarity and displacement ellipsoids are drawn at the 30% probability level. | ||
| 1 | 2 | 3 | |
|---|---|---|---|
| a (30)*, (34)* compound 3 C atom labelling. | |||
| M1–Bi1 | 2.7549(6) | 2.6959(3) | 2.8535(2) |
| M1–C30(34)* | 1.9716(18) | 1.980(3) | 2.197(3) |
| M1–N1 | 1.9262(15) | 2.012(2) | 2.212(2) |
| M1–N2 | 1.9250(14) | 1.997(2) | 2.210(2) |
| Bi1–C32 | 2.292(2) | 2.293(3) | 2.280(3) |
| Bi1–C34(30)* | 2.2914(19) | 2.282(3) | 2.283(3) |
| N1–M1–N2 | 95.84(6) | 93.39(9) | 86.72(7) |
| Bi1–M1–C30(34)* | 109.56(6) | 113.47(8) | 133.43(7) |
| C32–Bi1–C34(30)* | 93.29(8) | 93.63(12) | 92.93(13) |
| C34(30)*–Bi1–M1 | 93.33(5) | 94.66(9) | 95.19(10) |
| C32–Bi1–M1 | 92.72(5) | 92.83(8) | 95.50(9) |
| M1–N1–C1–C3 | 18.91 | 17.66 | −25.15 |
1 and 2 crystallize in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and 3 in the monoclinic P21/n. Each of the group 13 metals in 1 to 3 adopts a distorted tetrahedral geometry, whereas the Bi atom shows a pyramidal coordination sphere. As was pointed out by Power, there is a possibility for the conjugation of the lone pair of Bi with the empty orbital on the group 13 elements (Al, Ga and In).10 However, due to its large inversion barrier and the high s-orbital contribution of the electron lone pair, the Bi atom adopts a pyramidal coordination sphere without any π-interaction. The C3N2M rings in the starting reagents RM are planar, whereas the metal atoms M in 1–3 are out of plane (deviation from the best plane of the ligand backbone 0.6097(18) Å 1, 0.616(3) Å 2, 0.876(3) Å 3). The bite angles of the chelating organic ligand R are 95.84(6)° (1), 93.39(9)° (2) and 86.72(7)° (3), respectively. The two independent M–N distances in 1–3 are virtually the same [1.9250(14), 1.9262(15) (1); 1.997(2), 2.012(2) (2); 2.210(2), 2.212(2) (3)] and the Bi–M–Cethyl angles of 1–3 are 109.56(6)°, 113.47(8)° and 133.43(7)°, respectively. The M–Bi bond distances [2.7549(6) (1); 2.6959(3) (2); 2.8535(2) (3)] are comparable to the sum of the respective covalent radii (covalent radii of Al = 1.26 Å; Ga = 1.24 Å; In = 1.42 Å; Bi = 1.51 Å)11 and agree well with those observed in M–E σ-bonded trimers [Me2MBi(SiMe3)2]3 (M = Al, 2.774 Å; Ga, 2.762 Å; In, 2.915 Å) and the monomers [(dmap)R2AlBi(SiMe3)2] (R = Me, 2.755(2) Å; Et, 2.750(2) Å; dmap = 4-dimethylamino pyridine).12
and 3 in the monoclinic P21/n. Each of the group 13 metals in 1 to 3 adopts a distorted tetrahedral geometry, whereas the Bi atom shows a pyramidal coordination sphere. As was pointed out by Power, there is a possibility for the conjugation of the lone pair of Bi with the empty orbital on the group 13 elements (Al, Ga and In).10 However, due to its large inversion barrier and the high s-orbital contribution of the electron lone pair, the Bi atom adopts a pyramidal coordination sphere without any π-interaction. The C3N2M rings in the starting reagents RM are planar, whereas the metal atoms M in 1–3 are out of plane (deviation from the best plane of the ligand backbone 0.6097(18) Å 1, 0.616(3) Å 2, 0.876(3) Å 3). The bite angles of the chelating organic ligand R are 95.84(6)° (1), 93.39(9)° (2) and 86.72(7)° (3), respectively. The two independent M–N distances in 1–3 are virtually the same [1.9250(14), 1.9262(15) (1); 1.997(2), 2.012(2) (2); 2.210(2), 2.212(2) (3)] and the Bi–M–Cethyl angles of 1–3 are 109.56(6)°, 113.47(8)° and 133.43(7)°, respectively. The M–Bi bond distances [2.7549(6) (1); 2.6959(3) (2); 2.8535(2) (3)] are comparable to the sum of the respective covalent radii (covalent radii of Al = 1.26 Å; Ga = 1.24 Å; In = 1.42 Å; Bi = 1.51 Å)11 and agree well with those observed in M–E σ-bonded trimers [Me2MBi(SiMe3)2]3 (M = Al, 2.774 Å; Ga, 2.762 Å; In, 2.915 Å) and the monomers [(dmap)R2AlBi(SiMe3)2] (R = Me, 2.755(2) Å; Et, 2.750(2) Å; dmap = 4-dimethylamino pyridine).12
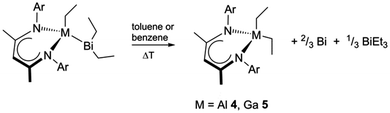
1–3 further react at elevated temperatures upon activation of the second ethyl group at the Bi atom and the quantitative formation of RMEt2 (E = Al 4; Ga 5), BiEt3 and elemental bismuth (Scheme 2) occurs. For instance, pure 1 and 2 react in toluene solution upon heating at 90 °C for 5 h (4) and 120 °C for 15 h (5), respectively. EDX and PXRD analyses of the solid precipitates confirm the formation of the Bi metal (Fig. S16 and S17, ESI‡). The 1H NMR spectra of the reaction mixtures show the presence of BiEt3 and 4 or 5, respectively, in molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. S13 and S14, ESI‡). Although RInEt(BiEt2) (3) also follows the same reductive pathway in the beginning, the sensitivity of RIn toward heat and light makes the reaction pathway more complex. RInEt2 is initially formed, but further decomposition reaction occurs.
3 (Fig. S13 and S14, ESI‡). Although RInEt(BiEt2) (3) also follows the same reductive pathway in the beginning, the sensitivity of RIn toward heat and light makes the reaction pathway more complex. RInEt2 is initially formed, but further decomposition reaction occurs.
5 was isolated in a large-scale experiment and fully characterized using 1H and 13C NMR spectroscopy and single-crystal X-ray diffraction. The molecular structure of 5 (Fig. S21, ESI‡) is closely related to the analogous derivatives, RGaX2 (X = Cl, I, Me).13 The formation of 1/3 equivalent of BiEt3 with respect to 4 and 5, respectively, as well as Bi metal in the reaction mixture apparently shows the formation of a redox-active low-valent [BiEt]x reaction intermediate, which consequently undergoes disproportionation at high temperature (BiEt → 2/3Bi + 1/3BiEt3). Similar reductive elimination of Cp*H has been reported for Cp*2AlH and Cp*AlH2 (Cp* = C5Me5).14 Furthermore, RGa was shown to undergo comparable insertion reactions with group 13 and group 14 alkyl complexes.15 However, the controlled decomposition of (hetero)multimetallic complexes into low valent metal complexes – a mechanism following Schnöckel's route in the synthesis of metalloid Al and Ga cluster complexes using metastable AlI and GaI halides – is still very rare.
The selective activation of Bi–C bonds by low-valent group 13 complexes RM (M = Al, Ga, In) was established as new synthetic method for the synthesis of intermetallic compounds of heavier main-group metals containing a metal–metal bond. The specific reaction sequence occurs with the formation of stable intermediates of the type RM(Et)BiEt21–3, which upon heating react leading to the formation of the low-valent [BiEt]x species. The reaction has a promising potential for the general synthesis of similar intermetallic complexes through consecutive bond activations. 1–3 are rare examples of structurally characterized heavier main-group 13/15 σ-bonded derivatives and the controlled disproportionation reaction of the low-valent [BiEt]x intermediate in the presence of stabilizing agents is currently under active investigation.
Notes and references
- (a) C. Dohmeier, C. Robl, M. Tacke and H. Schnöckel, Angew. Chem., 1991, 103, 594 ( Angew. Chem., Int. Ed. Engl. , 1991 , 30 , 564 ) CrossRef CAS PubMed; (b) W. Hiller, K.-W. Klinkhammer, W. Uhl and J. Wagner, Angew. Chem., 1991, 103, 182 ( Angew. Chem., Int. Ed. Engl. , 1991 , 30 , 179 ) CrossRef CAS PubMed.
- (a) G. Linti and H. Schnöckel, Coord. Chem. Rev., 2000, 206–207, 285 CrossRef CAS; (b) A. Schnepf and H. Schnöckel, Angew. Chem., 2002, 114, 3682 ( Angew. Chem., Int. Ed. , 2002 , 41 , 3532 ) CrossRef; (c) M. Huber, A. Schnepf, C. E. Anson and H. Schnöckel, Angew. Chem., 2008, 120, 8323 ( Angew. Chem., Int. Ed. , 2008 , 47 , 8201 ) CrossRef PubMed and references therein.
- (a) O. T. Beachley Jr., M. R. Churchill, J. C. Fettinger, J. C. Pazik and L. Victoriano, J. Am. Chem. Soc., 1986, 108, 4666 CrossRef; (b) W. Uhl, W. Hiller, M. Layh and W. Schwarz, Angew. Chem., 1992, 104, 1378 ( Angew. Chem., Int. Ed. Engl. , 1992 , 31 , 1364 ) CrossRef CAS PubMed; (c) W. Uhl, R. Graupner, M. Layh and U. Schütz, J. Organomet. Chem., 1995, 493, C1 CrossRef CAS; (d) G. Linti, J. Organomet. Chem., 1996, 520, 107 CrossRef CAS; (e) N. J. Hardman, B. E. Eichler and P. P. Power, Chem. Commun., 2000, 1991 RSC; (f) C. Cui, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, H. Hao and F. Cimpoesu, Angew. Chem., 2000, 112, 4444 ( Angew. Chem., Int. Ed. , 2000 , 39 , 4274 ) CrossRef; (g) P. Jutzi and L. O. Schebaum, J. Organomet. Chem., 2002, 654, 176 CrossRef CAS; (h) N. J. Hardman, R. J. Wright, A. D. Phillips and P. P. Power, J. Am. Chem. Soc., 2003, 125, 2667 CrossRef CAS PubMed; (i) M. S. Hill and P. B. Hitchcock, Chem. Commun., 2004, 1818 RSC; (j) Y. Wang and G. H. Robinson, Organometallics, 2007, 26, 2 CrossRef CAS; (k) M. Asay, C. Jones and M. Driess, Chem. Rev., 2011, 111, 354 CrossRef CAS PubMed.
- (a) C. Cui, H. W. Roesky, H.-G. Schmidt and M. Noltemeyer, Angew. Chem., 2000, 112, 4705 ( Angew. Chem., Int. Ed. , 2000 , 39 , 4531 ) CrossRef; (b) N. J. Hardman, C. Cui, H. W. Roesky, W. H. Fink and P. P. Power, Angew. Chem., 2001, 113, 2230 ( Angew. Chem., Int. Ed. , 2001 , 40 , 2172 ) CrossRef; (c) N. J. Hardman and P. P. Power, Inorg. Chem., 2001, 40, 2474 CrossRef CAS; (d) Y. Peng, H. Fan, H. Zhu, H. W. Roesky, J. Magull and C. E. Hughes, Angew. Chem., 2004, 116, 3525 ( Angew. Chem., Int. Ed. , 2004 , 43 , 3443 ) CrossRef PubMed; (e) Y. Peng, H. Fan, V. Jancik, H. W. Roesky and R. Herbst-Irmer, Angew. Chem., 2004, 116, 6316 ( Angew. Chem., Int. Ed. , 2004 , 43 , 6190 ) CrossRef PubMed; (f) H. Zhu, J. Chai, A. Stasch, H. W. Roesky, T. Blunck, D. Vidovic, J. Magull, H.-G. Schmidt and M. Noltemeyer, Eur. J. Inorg. Chem., 2004, 4046 CrossRef CAS PubMed; (g) H. Zhu, J. Chai, V. Jancik, H. W. Roesky, W. A. Merrill and P. P. Power, J. Am. Chem. Soc., 2005, 127, 10170 CrossRef CAS PubMed; (h) H. Zhu, Y. Chai, H. Fan, H. W. Roesky, U. N. Nehete, H.-G. Schmidt and M. Noltemeyer, Eur. J. Inorg. Chem., 2005, 2147 CrossRef CAS PubMed; (i) H. Zhu, J. Chai, H. Fan, H. W. Roesky, C. He, V. Jancik, H.-G. Schmidt, M. Noltemeyer, W. A. Merill and P. P. Power, Angew. Chem., 2005, 117, 5220 ( Angew. Chem., Int. Ed. , 2005 , 44 , 5090 ) CrossRef PubMed; (j) H. Zhu, R. B. Oswald, H. Fan, H. W. Roesky, Q. Ma, Z. Yang, H.-G. Schmidt, M. Noltemeyer, K. Starke and N. S. Hosmane, J. Am. Chem. Soc., 2006, 128, 5100 CrossRef CAS PubMed; (k) X. Li, X. Cheng, H. Song and C. Cui, Organometallics, 2007, 26, 1039 CrossRef CAS; (l) G. Prabusankar, A. Doddi, C. Gemel, M. Winter and R. A. Fischer, Inorg. Chem., 2010, 49, 7976 CrossRef CAS PubMed; (m) J. Li, X. Li, W. Huang, H. Hu, J. Zhang and C. Cui, Chem. – Eur. J., 2012, 18, 15263 CrossRef CAS PubMed.
- (a) R. A. Fischer and J. Weiß, Angew. Chem., 1999, 111, 3002 ( Angew. Chem., Int. Ed. , 1999 , 38 , 2830 ) CrossRef; (b) C. Gemel, T. Steinke, M. Cokoja, A. Kempter and R. A. Fischer, Eur. J. Inorg. Chem., 2004, 4161 CrossRef CAS PubMed; (c) S. González-Gallardo, T. Bollermann, R. A. Fischer and R. Murugavel, Chem. Rev., 2012, 112, 3136 CrossRef PubMed.
- (a) J. D. Gorden, A. Voigt, C. L. B. Macdonald, J. S. Silverman and A. H. Cowley, J. Am. Chem. Soc., 2000, 122, 950 CrossRef CAS; (b) J. D. Gorden, C. L. B. Macdonald and A. H. Cowley, Chem. Commun., 2001, 75 RSC; (c) N. J. Hardman, P. P. Power, J. D. Gorden, C. L. B. Macdonald and A. H. Cowley, Chem. Commun., 2001, 1866 RSC; (d) Z. Yang, X. Ma, R. B. Oswald, H. W. Roesky, H. Zhu, C. Schulzke, K. Starke, M. Baldus, H.-G. Schmidt and M. Noltemeyer, Angew. Chem., 2005, 117, 7234 ( Angew. Chem., Int. Ed. , 2005 , 44 , 7072 ) CrossRef PubMed; (e) J. D. Gorden, C. L. B. Macdonald and A. H. Cowley, Main Group Chem., 2005, 4, 33 CrossRef CAS; (f) S. Schulz, A. Kuczkowski, D. Schuchmann, U. Flörke and M. Nieger, Organometallics, 2006, 25, 5487 CrossRef CAS.
- (a) S. Schulz, S. Heimann, A. Kuczkowski, D. Bläser and C. Wölper, Organometallics, 2013, 32, 3391 CrossRef CAS; (b) S. Heimann, S. Schulz, D. Bläser and C. Wölper, Eur. J. Inorg. Chem., 2013, 4909 CAS; (c) S. Heimann, D. Bläser, C. Wölper and S. Schulz, Organometallics, 2014, 33, 2295 CrossRef CAS.
- For a review article see: S. Schulz, Adv. Organomet. Chem., 2003, 49, 225 CrossRef CAS.
- S. Singh, H.-J. Ahn, A. Stasch, V. Jancik, H. W. Roesky, A. Pal, M. Biadene, R. Herbst-Irmer, M. Noltemeyer and H.-G. Schmidt, Inorg. Chem., 2006, 45, 1853 CrossRef CAS PubMed.
- (a) P. P. Power, Chem. Rev., 1999, 99, 3463 CrossRef CAS PubMed; (b) R. C. Fischer and P. P. Power, Chem. Rev., 2010, 110, 3877 CrossRef CAS PubMed.
- P. Pyykkö and M. Atsumi, Chem. – Eur. J., 2009, 15, 186 CrossRef PubMed.
- (a) S. Schulz and M. Nieger, Angew. Chem., 1999, 111, 1020 ( Angew. Chem., Int. Ed. , 1999 , 38 , 967 ) CrossRef; (b) A. Kuczkowski, F. Thomas, S. Schulz and M. Nieger, Organometallics, 2000, 19, 5758 CrossRef CAS; (c) F. Thomas, S. Schulz and M. Nieger, Organometallics, 2002, 21, 2793 CrossRef CAS; (d) F. Thomas, S. Schulz, H. Mansikkamäki and M. Nieger, Angew. Chem., 2003, 115, 5800 ( Angew. Chem., Int. Ed. , 2003 , 42 , 5641 ) CrossRef PubMed.
- M. Stender, B. E. Eichler, N. J. Hardman, P. P. Power, J. Prust, M. Noltemeyer and H. W. Roesky, Inorg. Chem., 2001, 40, 2794 CrossRef CAS PubMed.
- C. Ganesamoorthy, S. Loerke, C. Gemel, P. Jerabek, M. Winter, G. Frenking and R. A. Fischer, Chem. Commun., 2013, 49, 2858 RSC.
- (a) A. Kempter, C. Gemel and R. A. Fischer, Inorg. Chem., 2008, 47, 7279 CrossRef CAS PubMed; (b) G. Prabusankar, C. Gemel, M. Winter, R. W. Seidel and R. A. Fischer, Chem. – Eur. J., 2010, 16, 6041 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to Prof. M. Jansen on the occasion of his 70th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedure and characterization of 1–5 including crystallographic data for 1–3 and 5. CCDC 1011108 (1), 1011107 (2), 1011105 (3), and 1011106 (5). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc05028b |
| This journal is © The Royal Society of Chemistry 2014 |