Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic constructive deoxygenation of lignin-derived phenols: new C–C bond formation processes from imidazole-sulfonates and ether cleavage reactions†
Stuart M.
Leckie
,
Gavin J.
Harkness
and
Matthew L.
Clarke
*
School of Chemistry, University of St Andrews, EaStCHEM, St Andrews, Fife, UK. E-mail: mc28@st-andrews.ac.uk; Fax: +44 (0)1334 463808; Tel: +44 (0)1334 463850
First published on 12th August 2014
Abstract
As part of a programme aimed at exploiting lignin as a chemical feedstock for less oxygenated fine chemicals, several catalytic C–C bond forming reactions utilising guaiacol imidazole sulfonate are demonstrated. These include the cross-coupling of a Grignard, a non-toxic cyanide source, a benzoxazole, and nitromethane. A modified Meyers reaction is used to accomplish a second constructive deoxygenation on a benzoxazole functionalised anisole.
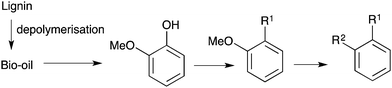
The production of cellulose-derived chemicals is significantly more commercially attractive if economic value can be obtained from the lignin fraction of ligno-cellulose. There is currently great interest in researching the conversion of lignin to aromatics and alkanes.1 These studies generally focus on the possible production of fuels, bulk, or commodity chemicals. The reactions used are depolymerisation of lignin, and hydro-deoxygenation reactions i.e. the replacement of the C–O bond with inert C–H bonds.2,3
We considered a new challenge in this field of renewable chemistry; if a small portion of lignin-derived bio-oils can be converted into one or more higher value fine chemicals, prior to hydro-deoxygenation, then extra economic value can be derived from this lignin fraction. The research to find efficient lignin depolymerisation methods is still very much an expanding effort. None-the-less in the research published so far, 2-methoxyphenol (guaiacol) is a very common major component in lignin-derived bio-oils.3 2-Methoxyphenol is somewhat more volatile than some aromatic components, and can also be converted during processing to catechol, which may be possible to separate due to its acidity. While other building blocks may become viable in the future, it seems likely that 2-methoxyphenol and catechol will be produced from lignin feedstocks.2b,3 Another possibility is that catalysis chemistry could be developed to selectively remove guaiacol or other monomers from lignin.4 A further speculative possibility is to functionalise specific monomers in a bio-oil mixture, to give new fine chemicals that are readily separated from the rest of the bio-oil.
In order to give a larger range of possible target fine chemicals, new catalytic chemistry needs to be developed to convert chemicals like 2-methoxyphenol into less oxygenated, but still functionalised aromatic compounds, i.e. the challenge of catalytic constructive deoxygenation (Scheme 1). Longer term requirements are likely to be heavily focused on cost, so while improving the economics of the catalytic processes needs to be addressed in due course, certain aspects such as the reagents used to activate C–O bonds and the processes chosen to study need to considered now. This actually leads to some interesting problems for catalysis chemists to study. Here we show the first studies on this concept and report new protocols to replace one or both C–O bonds in 2-methoxyphenol with C–C bonds.
The conversion of phenolic derivatives to activated compounds, followed by cross-coupling reactions is, of course, known methodology in a general sense. However, specific methods need to be developed for 2-methoxyphenol that do not use expensive triflates or other incompatible or expensive reagents.
We have focused on the coupling of the imidazole-sulfonate of 2-methoxyphenol, 1 (Scheme 2) since this is a reasonably cheap leaving group that is also claimed to give less toxic waste streams relative to triflates and their derivatives.5 For the cross-coupling partners, we have assessed a range of suitable possibilities, but here we have studied Grignard reagents, nitromethane, heteroaromatic compounds and the cyanide anion since these are economic coupling partners.
 | ||
| Scheme 2 Catalytic reactions of 2-methoxyphenyl-1H-imidazole-1-sulfonate with Grignards, nitromethane and benzoxazole. | ||
The Kumada cross-coupling of Grignard reagents with imidazole-sulfonates had not been reported, but our starting point was procedures that work well for aryl halides. The use of [PdCl2(dppf)] (dppf = 1,1′-bisdiphenylphosphino-ferrocene) in methyl-THF has previously been found to be an excellent procedure for Grignard cross-coupling, even under very concentrated conditions.6 However, none of the desired product was formed. Changing solvent to tert-amyl methyl ether enabled the cross coupling to proceed, although very unselectively, and very slowly using [PdCl2(dppf)] (see ESI†). We were pleased to find that the use of [PdCl2((S)-Xyl-phanephos)]7 as catalyst is much more active and selective (Scheme 2, eqn (1)).
Excellent results were obtained using this previously unexplored Grignard coupling catalyst. In the ESI† a further table of results comparing Pd/dppf and Pd/Xyl-phanephos for aryl halides shows that PdCl2(Xyl-phanephos) is a more active catalyst than the widely applied Pd/dppf catalyst for this reaction. We used the expensive enantiomerically pure catalyst, but the racemic analogue would be a relatively economic ligand to use in achiral C–C bond forming reactions. Xyl-phanephos has a larger bite angle than dppf; this is generally associated with more efficient reductive elimination, but a lower propensity to other off-cycle events using this system is also possible.
The coupling of nitromethane with imidazole-sulfonates was not known, but there had been a report of coupling aryl halides with nitromethane.8 This protocol makes use of Pd/XPhos as the leading catalyst, although none of these operates at low catalyst loading. Our initial screening (see ESI† and Scheme 2, eqn (2)) revealed that the combination used for aryl halides was ineffective, but Pd/TrixiePhos proved to be the only reasonably effective catalyst for coupling nitromethane with 1 to give desired product 3. Further research on more active nitromethylation catalysts in general would be worthwhile. It seems likely a mono-ligated Pd centre is desirable given the very bulky ligands required for any conversion in this study. Another pro-nucleophile with relatively acidic C–H bonds is benzoxazole. In this case, there had already been a report of C–H functionalisation using a range of phenols activated as their imidazole sulfonates, including compound 1.9 We therefore used this procedure here (Scheme 2, eqn (3)), although again note that improvements in catalytic turnover are desirable in the future.
We next studied cyanation using cheap and non-toxic K4Fe(CN)6. This cyanide source, introduced in a seminal paper by Beller and co-workers, has been studied in the coupling with aryl halides and aryl mesylates, but not imidazole-sulfonates.10 A more extensive screening of conditions and many different catalysts can be found in the ESI;† most catalysts are not sufficiently active, and 1 slowly hydrolyses to guaiacol, 6. Table 1 shows that combinations of either X-Phos or triphenylphosphine combined with Pd(II) pre-catalysts were effective. The more economic triphenylphosphine based system was found to give the optimal results for the production of 5 (Table 1, entry 7).
| Entry | Pd precursor (mol%) | Ligand (mol%) | Temp. (°C) | Time (h) | Ratio 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6a 6a |
Yield (%) |
|---|---|---|---|---|---|---|
| a As judged by 1H NMR of the crude reaction mixture. Yields are pure product after chromatography. b 0.5 equiv. of K4FeCN6. c 0.21 equiv. of K4FeCN6. | ||||||
| 1b | Pd(OAc)2 (5 mol%) | X-Phos (10 mol%) | 110 | 72 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 |
| 2c | Pd(TFA)2 (1 mol%) | X-Phos (2 mol%) | 110 | 48 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
— |
| 3 | Pd(TFA)2 (1 mol%) | X-Phos (2 mol%) | 110 | 48 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
— |
| 4 | Pd(TFA)2 (1 mol%) | X-Phos (3 mol%) | 110 | 24 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
— |
| 5c | Pd(TFA)2 (1 mol%) | PPh3 (3 mol%) | 110 | 24 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
— |
| 6 | Pd(TFA)2 (1 mol%) | PPh3 (3 mol%) | 100 | 48 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
— |
| 7c | Pd(TFA)2 (1 mol%) | PPh3 (3 mol%) | 90 | 48 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
73 |
The cross-coupling processes shown above suggest that, beyond the realm of this specific project, it should be possible to carry out effective cyanation, Grignard cross-coupling and nitromethylation reactions using phenol-imidazolesulfonates and the new procedures identified here. Moreover, these studies show that, with further research, it should be feasible to develop scalable methods for the C–C bond forming reaction using 1. This should be useful for making various phenolic compounds containing only one aromatic C–O bond. To increase the potential scope of this building block, it would be desirable to be able to swap the remaining aromatic C–O bond for a C–C bond.11 There are some important fine chemicals that could be produced effectively using this type of route,12 but at this early stage, we wanted to map out what was possible.
As already noted, it is convenient to produce 4, using C–H activation coupling of benzoxazole with 1, so we considered modifying the Meyers reaction towards this class of substrate. The Meyers reaction normally uses certain oxazolines as activating groups for ether cleavage,13 and to the best of our knowledge, there are not any examples of Meyers coupling using this type of benzoxazole. We were pleased to find that these reactions proceed well at near ambient temperatures using a range of aromatic, alkenyl and alkyl Grignards. Scheme 3 lists the products 7a–7h produced and reaction conditions.
In summary, some cross-coupling reactions that use relatively economic nucleophilic partners and the imidazole-sulfonate of 2-methoxyphenol, 1 have been studied. It is proposed that this type of catalysis might be useful for creaming off some high value products from bio-oil mixtures, or bio-oil derived 2-methoxyphenol. In this case, we have identified several new protocols for cross-coupling imidazole sulfonate derivatives with Grignards, nitromethane and a non-toxic cyanide source. Modified Meyers reactions on benzoxazoles are also reported. These discoveries should prove enabling to those needing new organic methodology, in addition to presenting the first steps towards constructive deoxygenation reactions of renewables.
We thank the EPSRC for funding, and all the technical staff in the School of Chemistry for their assistance.
Notes and references
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552 CrossRef CAS PubMed.
- (a) R. C. Runnebaum, T. Nimmanwudipong, D. E. Block and B. C. Gates, Catal. Sci. Technol., 2012, 2, 113 RSC; (b) M. P. Pandey and C. S. Kim, Chem. Eng. Technol., 2011, 34, 29 CrossRef CAS PubMed.
- (a) C. Amen-Chen, H. Pakdel and C. Roy, Biomass Bioenergy, 1997, 13, 25 CrossRef CAS; (b) J. Zakzeski, A. L. Jongerius, P. C. A. Bruijnincx and B. M. Weckhuysen, ChemSusChem, 2012, 5, 1602 CrossRef CAS PubMed; (c) P. Varanasi, P. Singh, M. Auer, P. D. Adams, B. A. Simmons and S. Singh, Biotechnol. Biofuels, 2013, 6, 14 CrossRef CAS PubMed; (d) For oxidative cleavage, see R. Jastrzebski, B. M. Weckhuysen and P. C. A. Bruijnincx, Chem. Commun., 2013, 49, 6912 RSC.
- (a) A. G. Sergeev, J. D. Webb and J. F. Hartwig, J. Am. Chem. Soc., 2012, 134, 20226 CrossRef CAS PubMed; (b) T. vom Stein, T. Wiegard, C. Merkens, J. Klankermayer and W. Leitner, ChemCatChem, 2013, 5, 439 CrossRef CAS PubMed.
- (a) L. Ackermann, R. Sandmann and W. Song, Org. Lett., 2011, 13, 1784 CrossRef CAS PubMed; (b) J. F. Cívicos, D. A. Alonso and C. Nájera, Eur. J. Org. Chem., 2012, 3670 CrossRef PubMed; (c) J. Albaneze-Walker, R. Raju, J. A. Vance, A. J. Goodman, M. R. Reeder, J. Liao, M. T. Maust, P. A. Irish, P. Espino and D. R. Andrews, Org. Lett., 2009, 11, 1463 CrossRef CAS PubMed.
- (a) T. Hayashi, M. Konishi, Y. Kobori, M. Kumada, T. Higuchi and K. Hirotsu, J. Am. Chem. Soc., 1984, 106, 158 CrossRef CAS; (b) T. Hayashi, M. Konishi and M. Kumada, Tetrahedron Lett., 1979, 21, 1871 CrossRef; (c) E. J. Milton and M. L. Clarke, Green Chem., 2010, 12, 381 RSC.
- T. M. Konrad, J. A. Fuentes, A. M. Z. Slawin and M. L. Clarke, Angew. Chem., Int. Ed., 2010, 49, 9197 CrossRef CAS PubMed.
- (a) R. R. Walvoord, S. Berritt and M. C. Kozlowski, Org. Lett., 2012, 14, 4086 CrossRef CAS PubMed; (b) R. R. Walvoord and M. C. Kozlowski, J. Org. Chem., 2013, 78, 8859 CrossRef CAS PubMed.
- L. Ackermann, S. Barfusser and J. Pospech, Org. Lett., 2010, 12, 724 CrossRef CAS PubMed.
- (a) T. Schareina, A. Zapf and M. Beller, Chem. Commun., 2004, 1388 RSC; (b) P. Anbarasan, T. Schareina and M. Beller, Chem. Soc. Rev., 2011, 40, 5049 RSC and references cited therein; (c) P. Y. Yeung, C. M. So, C. P. Lau and F. Y. Kwong, Angew. Chem., Int. Ed., 2010, 49, 8918 CrossRef CAS PubMed.
- (a) J. W. Dankwardt, Angew. Chem., Int. Ed., 2004, 43, 2428 CrossRef CAS PubMed; (b) M. Tobisu, T. Shimasaki and N. Chatani, Angew. Chem., Int. Ed., 2008, 47, 4866 CrossRef CAS PubMed.
- A particularly desired problem to solve would be the coupling of some form of para-tolyl nucleophile with guaiacol-derived 2-methoxybenzonitrile. Such a reaction gives 4′-methyl-[1,1′-biphenyl]-2-carbonitrile, a key building block for the production of Losartan. Such compounds of relatively high value and significant demand are particularly important targets for this type of approach.
- (a) J. Mortier, Curr. Org. Chem., 2011, 15, 2413 CrossRef CAS; (b) M. Reuman and A. I. Meyers, Tetrahedron, 1985, 41, 837 CrossRef CAS; (c) T. G. Gant and A. I. Meyers, Tetrahedron, 1994, 50, 2297 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Further tables of results. Full experimental details, NMR. See DOI: 10.1039/c4cc04939j |
| This journal is © The Royal Society of Chemistry 2014 |