 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Isomerisation of 1,4-dichlorobenzene using highly acidic alkali chloroaluminate melts†
J.
Messner
a,
P. S.
Schulz
a,
N.
Taccardi
a,
Sven
Kuhlmann
b and
P.
Wasserscheid
*a
aLehrstuhl für Chemische Reaktionstechnik der Universität Erlangen-Nürnberg (FAU), Egerlandstrasse 3, D-91058 Erlangen, Germany. E-mail: peter.wasserscheid@fau.de; Fax: +49 9131 85 27421; Tel: +49 9131 85 27420
bLANXESS Chemical (China) Co. Ltd., 5 Corporate Avenue, No. 150 Hubin Road, HuangPu District, Shanghai, 200021, P. R. China
First published on 1st August 2014
Abstract
The isomerisation reaction of 1,4-dichlorobenzene leading to the thermodynamically favoured and technically desired 1,3-dichlorobenzene has been studied comparing highly acidic chloroaluminate melts with organic imidazolium and alkali metal ions. Interestingly, the inorganic melts show much higher reactivity and full recyclability if small AlCl3 losses are compensated and the reaction is carried out under slight HCl pressure.
Traditionally, Lewis acid catalysed reactions suffer from the need of hydrolysis for product isolation, causing the complete loss of the applied catalyst (e.g. AlCl3). Hence, liquid–liquid biphasic reaction systems with acidic ionic liquids are of great interest for more sustainable acid catalysis. Over the recent years, it has been demonstrated in a large number of publications1 that acidic ionic liquids allow a straightforward catalyst recycling by liquid phase separation, if none of the reactants form stable complexes with the acidic anions of the melt. Friedel–Crafts alkylations of aromatic compounds2,3 or alkane isomerisation reactions4 are among the prominent examples of this very successful strategy. Moreover, the physico-chemical properties of chloroaluminate ionic liquids, such as their melting points,5 viscosities6 and acidities,7 are well understood. In contrast, acid catalysis with acidic alkali chloroaluminate melts is much less developed. This is mainly due to the marked difference in physical properties, such as melting points. While imidazolium chloroaluminates, such as 1-ethyl-3-methylimidazolium chloride ([EMIM]Cl)–AlCl3, form liquid systems over a wide range of composition at room temperature,6 the respective LiCl–AlCl3 displays a melting point of 170 °C for χ(AlCl3) = 0.67.8 Despite these differences, anion speciation in both organic and inorganic chloroaluminates leads to quite a similar picture. Raman spectroscopy,9,10 mass spectroscopy (MS)11 and 27Al NMR spectroscopy12 have been applied to identify Cl−, [AlCl4]−, [Al2Cl7]− and [Al3Cl10]− in organic and inorganic chloroaluminate melts. For systems with a molar fraction χ(AlCl3) < 0.5 only Cl− and [AlCl4]− were found. Whereas [Al2Cl7]− and [Al3Cl10]− are the dominating species in melts with χ(AlCl3) > 0.5.13,14 However, in contrast to organic chloroaluminates (where only [AlCl4]− is found for χ(AlCl3) = 0.50),14 inorganic chloroaluminate melts have been shown to contain acidic [Al2Cl7]− species in equimolar chloride salt–AlCl3 mixtures.15 Simulation studies by Salanne et al.16 have confirmed this finding and claimed an increasing content of [Al2Cl7]− in inorganic chloroaluminate melts of equimolar composition by going from the potassium over the sodium to the lithium system. IR measurements resulted in additional evidence for this important difference between organic and inorganic chloroaluminate melts.17 However, although spectroscopically confirmed, this property was so far poorly exploited for superior performance in technically relevant catalytic transformations. For both organic and inorganic chloroaluminate systems a further increase of the acidity is possible by adding hydrogen chloride gas (HCl). As such, addition leads to the formation of super acids, the individual acidity of the melt system is a direct function of the chloride salt/AlCl3 ratio and the partial pressure of HCl gas.18,19
In this contribution we describe the use of acidic chloroaluminate melts, both with organic and inorganic cations, in the isomerisation reaction of 1,4-dichlorobenzene (1,4-DCB). The technical interest in this reaction originates from the fact that the chlorination of benzene with gaseous chlorine and using iron chloride as a catalyst leads typically to a kinetic product mixture of DCBs that contains much less 1,3-DCB than industrially needed. Due to the ortho-/para-directing effect of the primarily attached chloro substituent, the kinetic mixture contains mainly the 1,4- and 1,2-isomers and only small amounts of 1,3-DCB. However, 1,3-DCB is of high commercial interest as a feedstock for the synthesis of herbicides, insecticides, dyes and pharmaceuticals. Direct and selective synthesis routes to 1,3-DCB exist and are based on chlorination of 1,3-dinitrobenzene or on the Sandmeyer reaction of 3-chloroaniline. Both routes are much more expensive and much less atom efficient than direct benzene chlorination. Thus, the isomerisation of a kinetic DCB mixture to the 1,3-DCB-rich thermodynamic mixture is of significant technical interest. The thermodynamic equilibrium composition of DCB isomers at 150 °C is 60% 1,3-DCB, 32% 1,4-DCB and 8% 1,2-DCB.
The isomerisation of 1,4-DCB is not a new transformation. Olah et al.20 investigated this reaction in the 1960s using water promoted aluminium chloride as a catalyst. The isomerisation with AlCl3–water is highly selective to form only the three DCB isomers together with small amounts of polychlorinated biphenyls. However, in the homogeneous Olah protocol, the AlCl3 catalyst has to be hydrolysed for product isolation, leading to complete loss of the catalyst.
In this contribution we report on alternative liquid–liquid biphasic reaction systems for the isomerisation of DCB that allows easy product isolation and complete recycling of the highly acidic catalyst phase. For this purpose, we applied organic and inorganic, acidic chloroaluminate melts. In detail, we investigated the isomerisation of 1,4-DCB comparing AlCl3–[EMIM]Cl and AlCl3–MCl (MCl = LiCl or LiCl–NaCl) as reaction phases. As the DCB isomerisation requires strongly acidic conditions, all applied melts contained a molar ratio of χ(AlCl3) = 0.67. This was the maximal molar fraction of AlCl3 that allowed a reliable performance comparison between the different systems. In fact, the melting points of the AlCl3–LiCl system8 and the imidazolium system6 increase drastically above this composition.
In order to achieve comparable starting points for the reaction, all melts were prepared and heated to 170 °C prior to adding the 1,4-DCB substrate. In case of the reference system, 1,4-DCB was placed in a preheated flask at 170 °C and a mixture of AlCl3 and AlCl3·6H2O as a water donor was added directly to the organic component (for details of the experimental procedures see the ESI†). For the experiments shown in Fig. 1, samples were taken every hour, dissolved in dichloromethane, extracted with water and analysed by GC.
Most interestingly, there is a drastic difference in reactivity between the inorganic chloroaluminate melts and the imidazolium system. While the latter hardly shows any isomerisation activity under the applied conditions, the inorganic melts show significant activity and after a reaction time of 22 h exceed the level of conversion that was realized with the homogeneous Olah system20 (25% vs. 23% conversion). Remarkably, the Olah system shows very high reactivity in the first hour (19% conversion) while further reaction is very sluggish and only little extra conversion is observed in the following 23 hours. Comparing all chloroaluminate melts with the Olah reference system, it has to be kept in mind that all molten salt systems operate under liquid–liquid biphasic conditions while the AlCl3 is homogeneous in nature. In addition, it has to be stated that the Olah system was not easy to handle at a temperature of 170 °C due to AlCl3 sublimation and huge amounts of HCl gas evolving from the system. These problems were effectively minimized in all molten salt systems. In further experiments, we addressed the optimisation of the reaction conditions for the 1,4-DCB isomerisation using AlCl3–LiCl. In particular, we were interested in two aspects: (a) the influence of HCl on the isomerisation reaction (formed from traces of humidity in the LiCl); (b) the question whether recycling of the acidic melt is possible (saving large amounts of AlCl3 and avoid waste production from the AlCl3 hydrolysis).
In order to reveal the influence of dissolved HCl in the acidic molten salt, we compared the 1,4-DCB isomerisation reaction in an open flask with an otherwise identical reaction (4 h reaction time, 170 °C) in a glass autoclave (see the ESI† for experimental details) where all the formed HCl gas was kept in the system (Fig. 2).
 | ||
| Fig. 2 Isomerisation of 1,4-DCB with AlCl3–LiCl in an open flask compared to the reaction in a closed autoclave; 170 °C, 4 h, 20 mol% AlCl3 and 10 mol% LiCl. | ||
Very clearly, the isomerisation of 1,4-DCB was observed to proceed much more effectively in the autoclave experiment, with 1,3-DCB yields exceeding 45%. Crossing this conversion limit was found to be of practical relevance, as the DCB isomeric mixtures are liquids at room temperature with a molar fraction of the 1,3-isomer above 45%. Thus, after the reaction the liquid organic layer can be easily decanted from the now solid molten salt at room temperature.
For recycling, the resulting solid molten salt was re-melted and fresh 1,4-DCB was added. Two special precautions proved to be necessary to allow recycling with a constant yield of 1,3-DCB.
First, the AlCl3 content of the organic product phase was analysed by ICP to determine the level of Al-leaching. This level was found to be about 3% of the previously applied AlCl3. Replacing this loss was found to be beneficial for the catalyst recycling. Secondly, it turned out that recycling is much more effective if after the product removal – a process that requires the opening of the reactor under inert gas – 1 bar HCl-gas was added to the reaction system. Note that in an industrial scenario HCl is always omnipresent around an arene chlorination plant. With these two measures, the recycling of the acid catalyst phase worked indeed in an excellent manner with no loss of activity over at least five recycling runs (Fig. 3C). Thus, in comparison to the Olah system,21 the acidic lithium chloroaluminate system described here has three distinct advantages for DCB isomerisation: (a) drastic reduction of AlCl3 sublimation; (b) straightforward product isolation; and (c) re-use of more than 95 mol% of the applied AlCl3 in subsequent isomerisation reactions.
In order to evaluate the scope of the new catalytic system for the isomerisation of other dihaloarene compounds, we also studied the isomerisation of 1-bromo-4-chlorobenzene (BCB) (see the ESI† for details). It was found that the reaction of the BCB substrate required less harsh reaction conditions compared to the fully chlorinated substrate as expected from literature reports.21
Interestingly, the acidic [EMIM]+ chloroaluminate melt exhibits reasonable catalytic activity towards the isomerisation of BCB still being much less reactive than its inorganic counterpart (Fig. 4). While the AlCl3–LiCl system reached 40% of the 1,3 isomer after less than 1 hour of reaction time, the same final yield took 22 h with the [EMIM]+ melt of the same Al-content. A reasonable explanation for this marked difference in reactivity can be derived from the fact that the inorganic melt systems contain a higher concentration of acidic anions compared to the organic ones.
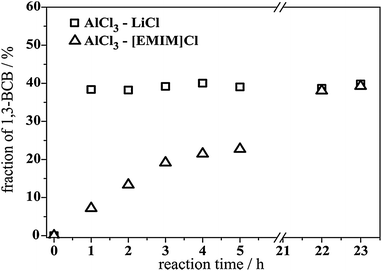 | ||
| Fig. 4 Isomerisation reaction of 1,4-BCB with AlCl3–LiCl vs. AlCl3–[EMIM]Cl (both XAlCl3 = 0.67); 170 °C, 4 h, 20 mol% AlCl3. | ||
In conclusion we could demonstrate that alkali chloroaluminate melts – and in particular lithium chloroaluminate melts – form highly active and recyclable catalyst phases for the technically relevant reaction of dihaloarene isomerisation.22 Remarkable differences in reactivity have been found between inorganic chloroaluminate systems and their imidazolium analogues at the same molar ratio of AlCl3. It has been further demonstrated that HCl partial pressure plays an important role in the catalyst recycling, and thus obviously in the reactivity of the isomerisation system. Replenishing AlCl3 losses and applying 1 bar HCl during the reaction resulted in a fully recyclable catalyst system for at least five dichlorobenzene isomerisation cycles.
The authors thank LANXESS Deutschland GmbH for financial support and for permission to publish this research.
Notes and references
- M. Earle, in Ionic Liquids in Synthesis, ed. T. Welton and P. Wasserscheid, Wiley-VCH, Weinheim, 2008 Search PubMed.
- S. J. Nara, J. R. Harjani and M. M. Salunkhe, J. Org. Chem., 2001, 66, 8616–8620 CrossRef CAS PubMed.
- J. A. Boon, J. A. Levisky, J. L. Pflug and J. S. Wilkes, J. Org. Chem., 1986, 51, 480–483 CrossRef CAS.
- C. Meyer and P. Wasserscheid, Chem. Commun., 2010, 46, 7625–7627 RSC.
- J. Estager, J. D. Holbrey and M. Swadzba-Kwasny, Chem. Soc. Rev., 2014, 43, 847–886 RSC.
- A. A. Fannin, Jr., D. A. Floreani, L. A. King, J. S. Landers, B. J. Piersma, D. J. Stech, R. L. Vaughn, J. S. Wilkes and J. L. Williams, J. Phys. Chem., 1984, 88, 2614–2621 CrossRef.
- J. Estager, A. A. Oliferenko, K. R. Seddon and M. Swadzba-Kwasny, Dalton Trans., 2010, 39, 11375–11382 RSC.
- R. A. Carpio, A. A. Fannin, Jr., F. C. Kibler, Jr., L. A. King and H. A. Oye, J. Chem. Eng. Data, 1983, 28, 34–36 CrossRef CAS.
- R. J. Gale, B. Gilbert and R. A. Osteryoung, Inorg. Chem., 1978, 17, 2728–2729 CrossRef CAS.
- S. J. Cyvin, P. Klaboe, E. Rytter and H. A. Øye, J. Chem. Phys., 1970, 52, 2776–2778 CrossRef CAS PubMed.
- G. Franzen, B. P. Gilbert, G. Pelzer and E. DePauw, Org. Mass Spectrom., 1986, 21, 443–444 CrossRef CAS PubMed.
- F. Taulelle and A. I. Popov, J. Solution Chem., 1986, 15, 463–471 CrossRef CAS.
- E. Rytter, H. A. Øye, S. J. Cyvin, B. N. Cyvin and P. Klaeboe, J. Inorg. Nucl. Chem., 1973, 35, 1185–1198 CrossRef CAS.
- H. A. Oye, M. Jagtoyen, T. Oksefjell and J. S. Wilkes, Mater. Sci. Forum, 1991, 73–75, 183–189 CrossRef CAS PubMed.
- R. S. Juvet, Jr., V. R. Shaw and M. A. Khan, J. Am. Chem. Soc., 1969, 91, 3788–3792 CrossRef.
- M. Salanne, L. J. A. Siqueira, A. P. Seitsonen, P. A. Madden and B. Kirchner, Condens. Matter, 2013, 1–12 Search PubMed.
- J. Hvistendahl, P. Klaeboe, E. Rytter and H. A. Oeye, Inorg. Chem., 1984, 23, 706–715 CrossRef CAS.
- G. P. Smith, A. S. Dworkin, R. M. Pagni and S. P. Zingg, J. Am. Chem. Soc., 1989, 111, 5075–5077 CrossRef CAS.
- G. P. Smith, A. S. Dworkin, R. M. Pagni and S. P. Zingg, J. Am. Chem. Soc., 1989, 111, 525–530 CrossRef CAS.
- G. A. Olah, W. S. Tolgyesi and R. E. A. Dear, J. Org. Chem., 1962, 27, 3449–3455 CrossRef CAS.
- G. A. Olah, W. S. Tolgyesi and R. E. A. Dear, J. Org. Chem., 1962, 27, 3455–3464 CrossRef CAS.
- S. Kuhlmann, U. Böger, P. Wasserscheid, S. Schlenk, J. Messner and H.-M. Weber, WO 2013/189848, Lanxess Deutschland GmbHChem. Abstr., 2013, 993608.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, ICP, GC, and GC-MS data. See DOI: 10.1039/c4cc04910a |
| This journal is © The Royal Society of Chemistry 2014 |


