 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spermidinium closo-dodecaborate-encapsulating liposomes as efficient boron delivery vehicles for neutron capture therapy†
Shoji
Tachikawa
ab,
Tatsuro
Miyoshi
b,
Hayato
Koganei
b,
Mohamed E.
El-Zaria
bc,
Clara
Viñas
d,
Minoru
Suzuki
e,
Koji
Ono
e and
Hiroyuki
Nakamura
*ab
aChemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8503, Japan. E-mail: hiro@res.titech.ac.jp; Fax: +81 45 924 5244
bDepartment of Chemistry, Faculty of Science, Gakushuin University, Mejiro, Toshima-ku, Tokyo 171-8588, Japan
cDepartment of Chemistry, Faculty of Science, Tanta University, 31527-Tanta, Egypt
dInstitut de Ciència de Materials de Barcelona (ICMAB-CSIC), Campus U.A.B., 08193 Bellaterra, Barcelona, Spain
eParticle Radiation Oncology Research Center, Kyoto University Research Reactor Institute, Asashiro-nishi, Kumatori-cho, Sennan-gun, Osaka 590-0494, Japan
First published on 21st August 2014
Abstract
closo-Dodecaborate-encapsulating liposomes were developed as boron delivery vehicles for neutron capture therapy. The use of spermidinium as a counter cation of closo-dodecaborates was essential not only for the preparation of high boron content liposome solutions but also for efficient boron delivery to tumors.
Boron neutron capture therapy (BNCT) has been attracting growing interest as one of the minimally invasive cancer therapies.1 Mercaptoundecahydrododecaborate (Na2[B12H11SH]; Na2BSH) and L-p-boronophenylalanine (L-BPA) have been used in BNCT for many years. L-BPA, in particular, has been widely used for the treatment of not only melanoma but also brain tumor2 and head and neck cancer3 because it can be taken up selectively by tumor cells through an amino acid transporter.4 The accelerator-based BNCT is now undergoing phase I clinical study for the treatment of brain tumor and head and neck cancer patients in Japan.5,6
In recent years, liposomal 10B carriers have attracted attention as some of the efficient boron delivery systems in BNCT.7–10 Several efficient in vivo BNCTs have been reported. Yanagië and coworkers demonstrated the first antitumor effect of Na2BSH-encapsulating liposomes conjugated with a monoclonal antibody specific for the carcinoembryonic antigen.9a,b Maruyama and co-workers developed transferrin-conjugating Na2BSH-encapsulating liposomes.9d,10 Although they succeeded in completely suppressing tumor growth in mice after neutron irradiation, the concentration of inner 10B of liposomes was limited in preparation due to osmotic reasons. For this reason, boron lipids embedded within the liposome bilayer have been studied.10–12 Hawthorne and coworkers developed liposomes incorporating Na3[1-(2-B10H9)-2-NH3B10H8] into the internal aqueous core and K[nido-7-CH3(CH2)15-7,8-C2B9H11] into the bilayer membrane to increase the boron content in liposomes.8b,13
We previously developed Na2BSH-encapsulating 10% distearoyl boron lipid (DSBL)11b liposomes that have high boron content with excellent boron delivery efficacy to tumors.14 In this communication, we studied the effects of the counter cations of boron clusters on liposome formation to develop high boron content liposomes for BNCT by overcoming osmotic pressure limitations.
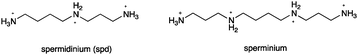
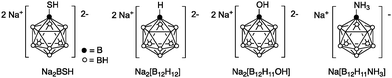
We selected three closo-dodecaborates, Na2[B12H12], Na2[B12H11OH]15 and Na[B12H11NH3]16 in addition to Na2BSH (Fig. 1). We first tested the cytotoxicity of the closo-dodecaborates toward colon 26 cells. The closo-dodecaborates are relatively non-toxic and the GI50 values of Na2[B12H12], Na2BSH, Na[B12H11NH3], and Na2[B12H11OH] are 5.1, 2.1, 32.9, and 7.7 mM, respectively (Table S1 in the ESI†). Liposomes containing the closo-dodecaborates were prepared from DSPC, cholesterol, and DSPE-PEG2000 by the reverse phase evaporation method with sizes of approximately 100 nm in diameter. The results are summarized in Table 1. The final boron and phosphorus concentrations of liposome solution containing Na2[B12H12] were 3438 ± 2.0 and 2864 ± 18.3 ppm, respectively, and the B/P ratio was 1.2 (entry 1). The higher B/P ratio indicates the higher boron content in liposomes. The liposome yield was 58% based on the total phospholipids used in the preparation. The B/P ratio of the liposome containing Na[B12H11NH3] was 2.2 (entry 2), which was slightly higher than those of liposomes containing Na2BSH, Na2[B12H11OH], and Na2[B12H12] (1.2–1.6, entries 1, 3, and 4). In the case of Na[B12H11NH3], an ammonium ion group served as one of the two counter cations of the closo-dodecaborate dianion. We speculated that ammonium counter cations would affect the encapsulation of closo-dodecaborates in liposomes. Recently, Gabel and coworkers reported that Na2BSH induces aggregation and membrane rupture, increasing wall thickness of the liposome and triggering the release of liposome contents.17 Indeed, it is known that tetramethylammonium (TMA) salts of closo-dodecaborates are insoluble in water, and the ion-exchange from Na2BSH to (TMA)2BSH proceeds readily, whereas the ion-exchange from (TMA)2BSH to Na2BSH is not easy. We predicted that encapsulation as well as liposome yield would be increased if we could reduce this interaction in the preparation of closo-dodecaborate-encapsulating liposomes. Thus we prepared various ammonium salts of BSH and examined their encapsulation into liposomes (entries 5–9 in Table 1). The B/P ratio of the liposome containing the n-C3H7NH3+ salt of BSH was similar to that of the liposome containing Na2BSH (entries 1 vs. 5). The H3N+C4H8NH3+ cation increased the B/P ratio (2.6) and boron concentration (4711 ppm). Interestingly, the B/P ratio dramatically increased to 3.4 when the spermidinium (spd) cation was employed (entry 7). In addition, the liposome yield was markedly increased to 98% and the final boron concentration of the liposome solution reached 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 867 ppm. In contrast, the B/P ratio of liposome containing (sperminium) BSH dropped to 2.7, although the liposome yield was still high (87% yield) and the final boron concentration of the liposome solution was high at 9759 ppm (entry 8). Liposome containing spd-[B12H11NH3] showed the highest B/P ratio (3.5); the boron concentration of the liposome solution reached 13
867 ppm. In contrast, the B/P ratio of liposome containing (sperminium) BSH dropped to 2.7, although the liposome yield was still high (87% yield) and the final boron concentration of the liposome solution was high at 9759 ppm (entry 8). Liposome containing spd-[B12H11NH3] showed the highest B/P ratio (3.5); the boron concentration of the liposome solution reached 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 ppm and the liposome yield was 95% (entry 9).
790 ppm and the liposome yield was 95% (entry 9).
| Entry | Boron cluster | B conc.b,c (ppm) | P conc. (ppm) | B/Pb,c | Yieldd (%) |
|---|---|---|---|---|---|
a In all cases, liposomes were prepared from DSPC, cholesterol, and DSPE-PEG2000 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11, molar ratio) by the REV method.
b Data are expressed as means ± standard deviation (SD).
c Boron and phosphorus concentrations of liposome solution were determined by ICP-AES.
d Liposome yields were calculated from the phosphorus concentration of liposome solution based on the total phospholipids used in preparation. 0.11, molar ratio) by the REV method.
b Data are expressed as means ± standard deviation (SD).
c Boron and phosphorus concentrations of liposome solution were determined by ICP-AES.
d Liposome yields were calculated from the phosphorus concentration of liposome solution based on the total phospholipids used in preparation.
|
|||||
| 1 | Na2BSH | 3438 ± 2.0 | 2864 ± 18.3 | 1.2 | 58 |
| 2 | Na[B12H11NH3] | 4072 ± 22.8 | 1835 ± 38.5 | 2.2 | 44 |
| 3 | Na2[B12H11OH] | 2635 ± 184.2 | 1600 ± 99.0 | 1.5 | 39 |
| 4 | Na2[B12H12] | 3133 ± 10.3 | 1932 ± 13.3 | 1.6 | 47 |
| 5 | (n-C3H7NH3)2BSH | 2874 ± 47.7 | 2225 ± 15.8 | 1.3 | 54 |
| 6 | (H3NC4H8NH3)BSH | 4711 ± 17.4 | 1833 ± 43.4 | 2.6 | 44 |
| 7 | spd-BSH | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 867 ± 185.8 867 ± 185.8 |
4046 ± 18.3 | 3.4 | 98 |
| 8 | (Sperminium)BSH | 9759 ± 139.6 | 3559 ± 44.5 | 2.7 | 87 |
| 9 | spd-[B12H11NH3] | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 970 ± 216.5 970 ± 216.5 |
3943 ± 43.4 | 3.5 | 95 |
|
|||||
We examined whether the formation of high boron content liposomes is affected by the viscosity of the closo-dodecaborate solutions. However, the spd cation of [B12H12]2− does not affect the viscosity of internal aqueous solution of liposomes (Table S3, ESI†). We measured liposome yields and B/P ratios under the condition of various ratios of BSH to spd cations (Na2BSH: [spermidine + HCl] = 1: X, X = 0, 0.25, 0.5, 1, and 2). As shown in Fig. 2, the B/P ratio reached a maximum of 3.4 when the BSH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) spd ratio was 1
spd ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Liposome yields showed a similar tendency to B/P ratios. The highest liposome yield was observed at the BSH to spd cation ratio of 1
1. Liposome yields showed a similar tendency to B/P ratios. The highest liposome yield was observed at the BSH to spd cation ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Transmission electron microscopy (TEM) analysis of spd-BSH-encapsulating liposomes and Na2BSH-encapsulating liposomes was also carried out using Cryo-TEM (Fig. 3). It is notable that the liposomes interacted with each other in the case of Na2BSH-encapsulating liposomes, whereas the liposomes dispersed in solution without interacting with each other in the case of spd-BSH-encapsulating liposomes.
1. Transmission electron microscopy (TEM) analysis of spd-BSH-encapsulating liposomes and Na2BSH-encapsulating liposomes was also carried out using Cryo-TEM (Fig. 3). It is notable that the liposomes interacted with each other in the case of Na2BSH-encapsulating liposomes, whereas the liposomes dispersed in solution without interacting with each other in the case of spd-BSH-encapsulating liposomes.
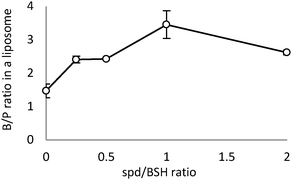 | ||
| Fig. 2 Effect of the amount of spd cation on spd-BSH encapsulation in liposomes. Boron/phosphorus (B/P) ratios of (spd)x-closo-dodecaborate-encapsulating liposomes are shown in the vertical axis. | ||
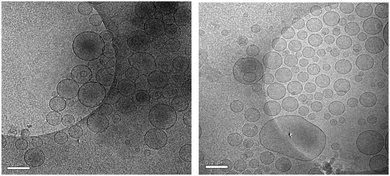 | ||
| Fig. 3 TEM images of Na2BSH-encapsulating liposomes (left) and spd-BSH-encapsulating liposomes (right). Scale bar represents 200 nm. | ||
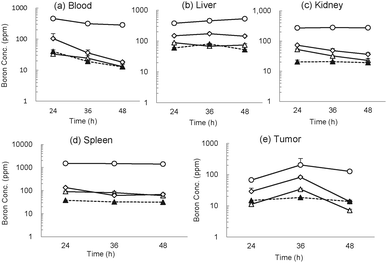
We next examined boron distribution of the spd-closo-dodecaborate-encapsulating liposomes in colon 26 tumor-bearing mice.18 The liposomes were injected at doses of 15, 30, and 100 mg [B] kg−1 body weight via the tail veins. Na2BSH- and Na2[B12H11NH3]-encapsulating liposomes were also injected at a dose of 30 mg [B] kg−1 as control experiments. The time courses of boron distribution in each organ are shown in Fig. 4. Blood boron concentrations of 460.7, 104.0, and 33.2 ppm were detected 24 h after injection of spd-BSH-encapsulating liposomes (100, 30, and 15 mg [B] kg−1), respectively (Fig. 4a). Blood boron concentration in mice injected with 100 mg [B] kg−1 of spd-BSH-encapsulating liposomes did not decrease notably during the 48 h period, whereas those in mice injected with 30 and 15 mg [B] kg−1 of spd-BSH-encapsulating liposomes gradually decreased in a time-dependent manner. The time courses of boron concentrations in liver, kidneys, and spleen are shown in Fig. 4b–d, respectively. Boron concentrations of 528.5, 144.2, and 74.4 ppm in liver were observed 48 h after the injection of 100, 30, and 15 mg [B] kg−1 of spd-BSH-encapsulating liposomes, respectively. In the meantime, maximum tumor boron concentrations of 202.7 and 82.4 ppm were achieved 36 h after injection at doses of 100 and 30 mg [B] kg−1, respectively. Even at the low boron dose of 15 mg [B] kg−1, the tumor boron concentration was 34.0 ppm at 36 h after injection (Fig. 4e). We also demonstrated the boron distribution of Na2BSH-encapsulating liposomes in tumor-bearing mice for comparison. Although blood and liver boron concentrations after injection of Na2BSH-encapsulating liposomes at a dose of 30 mg [B] kg−1 were similar to those after injection of spd-BSH-encapsulating liposomes at a dose of 30 mg [B] kg−1, kidney and spleen boron concentrations after injection of Na2BSH-encapsulating liposomes were lower than those after injection of spd-BSH-encapsulating liposomes up to 48 h. The tumor boron concentration at 36 h after injecting Na2BSH-encapsulating liposomes was 31.9 ppm, although the clearance of Na2BSH-encapsulating liposomes was slow (Fig. 4e).
A similar tendency was observed in spd-[B12H11NH3]-encapsulating liposomes (Fig. S1, ESI†). Blood, kidney, and spleen boron concentrations gradually decreased after injection. Maximum tumor boron concentrations of 242.2, 88.7, and 35.4 ppm were achieved 6 h after injection at doses of 100, 30, and 15 mg [B] kg−1, respectively. Interestingly, significant tumor boron accumulation was also observed in the case of Na[B12H11NH3]-encapsulating liposomes.
Finally, we examined the antitumor effect of liposomes containing spd closo-dodecaborates in colon 26 tumor bearing mice exposed to thermal neutron irradiation. Thermal neutron irradiation of the tumor-transplanted left thighs of mice was carried out 36 h after injection. The tumor growth curves of mice are shown in Fig. 5 (and in Fig. S2, ESI†). “Hot control (–●–)” and “Cold control (–×–)” represent tumor volumes of mice injected with saline with and without thermal neutron irradiation, respectively. Tumor growth was significantly suppressed in mice treated with spd-[10BSH]- and spd-[10B12H11NH3]-encapsulating liposomes at doses of 15, 30, and 100 mg [10B] kg−1 and exposed to thermal neutron irradiation. The tumor completely disappeared within three weeks even when a dose of 15 mg [10B] kg−1 was employed. Liposomes containing Na2[10BSH] and Na[10B12H11NH3] also inhibited tumor growth at a dose of 30 mg [10B] kg−1, and the tumor was completely controlled three weeks after thermal neutron irradiation. Tumor growth was suppressed in mice treated with Na2[10BSH] solution (100 mg [10B] kg−1) during the two weeks after thermal neutron irradiation. However, the tumor started to grow thereafter (Fig. 5). In contrast, tumor growth was not suppressed in mice treated with Na[10B12H11NH3] solution (100 mg [10B] kg−1) even after thermal neutron irradiation (Fig. S2, ESI†).
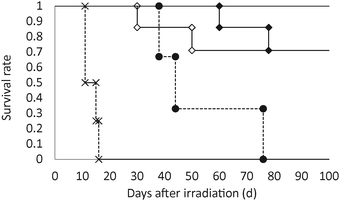
Fig. 6 shows the survival curve of tumor-bearing mice after thermal neutron irradiation. All untreated mice without thermal neutron irradiation died within two weeks. Thermal neutron irradiation enhanced mouse survival and all mice exposed to thermal neutron irradiation died within 78 days. Prolonged survival was observed in mice injected with spd-[10BSH]- and spd-[10B12H11NH3]-encapsulating liposomes; 72% of the mice that received a dose of 15 mg [10B] kg−1 survived up to 100 days after the thermal neutron irradiation. Furthermore, a remarkable antitumor effect was observed in the mice treated with spd-[10BSH]-encapsulating liposomes at a dose of 30 mg [10B] kg−1; 100% of the mice survived up to 100 days after the thermal neutron irradiation.
We succeeded in the preparation of high boron content liposomes. The use of spd as a counter cation of closo-dodecaborates was essential to obtain the liposomes with high yields and high B/P ratios. All of the mice injected with 30 mg [10B] kg−1 of spd-[10BSH]-encapsulating liposomes were completely cured while five of seven mice injected with 15 mg [10B] kg−1 of spd-[10BSH]- and spd-[10B12H11NH3]-encapsulating liposomes were cured 100 days after thermal neutron irradiation. The results indicate that the total amount of phospholipids could be reduced to less than one-seventh of those used to prepare Na2[10BSH]-encapsulating liposomes.19 We believe that the spd-closo-dodecaborate-encapsulating liposomes are promising candidates for clinical use in BNCT.
We thank Dr S. Kondoh (Teijin Pharm. Co. Ltd) for the measurement of osmotic pressures and Professor K. Iwata (Gakushuin University) for the measurement of the viscosity. This work was supported by Health and Labor Sciences Research Grants for research on Nanotechnical Medical from the Ministry of Health, Labor and Welfare (20100201) and the Ministry of Education, Science, Sports, Culture and Technology, Grant-in-Aid for Scientific Research (B) (No. 23390018) from Japan.
Notes and references
- (a) R. F. Barth, J. A. Coderre, M. G. Vicente and T. E. Blue, Clin. Cancer Res., 2005, 11, 3987 CrossRef CAS PubMed; (b) T. Yamamoto, K. Nakai and A. Matsumura, Cancer Lett., 2008, 262, 143 CrossRef CAS PubMed; (c) H. Hatanaka, J. Neurol., 1975, 209, 81 CrossRef CAS.
- S.-I. Miyatake, Y. Tamura, S. Kawabata, K. Iida, T. Kuroiwa and K. Ono, Neurosurgery, 2007, 61, 82 CrossRef PubMed.
- (a) I. Kato, K. Ono, Y. Sakurai, M. Ohmae, A. Maruhashi, Y. Imahori, M. Kirihata, M. Nakazawa and Y. Yura, Appl. Radiat. Isot., 2004, 61, 1069 CrossRef CAS PubMed; (b) T. Aihara, J. Hiratsuka, N. Morita, M. Uno, Y. Sakurai, A. Maruhashi, K. Ono and T. Harada, Head Neck, 2006, 28, 850 CrossRef PubMed; (c) L. Kankaanranta, T. Seppälä, H. Koivunoro, K. Saarilahti, T. Atula, J. Collan, E. Salli, M. Kortesniemi, J. Uusi-Simola, A. Mäkitie, M. Seppänen, H. Minn, P. Kotiluoto, I. Auterinen, S. Savolainen, M. Kouri and H. Joensuu, Int. J. Radiat. Oncol., Biol., Phys., 2007, 69, 475 CrossRef PubMed.
- (a) Y. Takahashi, Y. Imahori and K. Mineura, Clin. Cancer Res., 2003, 9, 5888 CAS; (b) A. Wittig, W. A. Sauerwein and J. A. Coderre, Radiat. Res., 2000, 153, 173 CrossRef CAS; (c) G. W. Kabalka and M. L. Yao, Anticancer Agents Med. Chem., 2006, 6, 111 CrossRef CAS; (d) Y. Imahori, Y. Ohmori, R. Fujii, K. Matsumoto and S. Ueda, Cancer Res., 1995, 55, 4225 CAS.
- M. Suzuki, H. Tanaka, Y. Sakurai, G. Kashino, L. Yong, S. Masunaga, Y. Kinashi, T. Mitsumoto, S. Yajima, H. Tsutsui, T. Sato, A. Maruhashi and K. Ono, Radiother. Oncol., 2009, 92, 89 CrossRef CAS PubMed.
- H. Tanaka, Y. Sakurai, M. Suzuki, T. Takata, S. Masunaga, Y. Kinashi, G. Kashino, Y. Liu, T. Mitsumoto, S. Yajima, H. Tsutsui, M. Takada, A. Maruhashi and K. Ono, Appl. Radiat. Isot., 2009, 67, S258 CrossRef CAS PubMed.
- H. Nakamura, Future Med. Chem., 2013, 5, 715 CrossRef CAS PubMed.
- For passive targeting, see: (a) K. Shelly, D. A. Feakes, M. F. Hawthorne, P. G. Schmidt, T. A. Krisch and W. F. Bauer, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 9039 CrossRef CAS; (b) D. A. Feakes, K. Shelly and M. F. Hawthorne, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 1367 CrossRef CAS.
- For active targeting, see: (a) H. Yanagië, T. Tomita, H. Kobayashi, Y. Fujii, T. Takahashi, K. Hasumi, H. Nariuchi and M. Sekiguchi, Br. J. Cancer, 1991, 63, 522 CrossRef; (b) H. Yanagië, T. Tomita, H. Kobayashi, Y. Fujii, Y. Nonaka, Y. Saegusa, K. Hasumi, M. Eriguchi, T. Kobayashi and K. Ono, Br. J. Cancer, 1997, 75, 660 CrossRef; (c) X. Q. Pan, H. Wang, S. Shukla, M. Sekido, D. M. Adams, W. Tjarks, R. F. Barth and R. J. Lee, Bioconjugate Chem., 2002, 13, 435 CrossRef CAS PubMed; (d) K. Maruyama, O. Ishida, S. Kasaoka, T. Takizawa, N. Utoguchi, A. Shinohara, M. Chiba, H. Kobayashi, M. Eriguchi and H. Yanagie, J. Controlled Release, 2004, 98, 195 CrossRef CAS PubMed.
- Y. Miyajima, H. Nakamura, Y. Kuwata, J.-D. Lee, S. Masunaga, K. Ono and K. Maruyama, Bioconjugate Chem., 2006, 17, 1314 CrossRef CAS PubMed.
- (a) H. Nakamura, Y. Miyajima, T. Takei, S. Kasaoka and K. Maruyama, Chem. Commun., 2004, 1910 RSC; (b) J.-D. Lee, M. Ueno, Y. Miyajima and H. Nakamura, Org. Lett., 2007, 9, 323 CrossRef CAS PubMed; (c) M. Ueno, H. S. Ban, K. Nakai, R. Inomata, Y. Kaneda, A. Matsumura and H. Nakamura, Bioorg. Med. Chem., 2010, 18, 3059 CrossRef CAS PubMed.
- (a) T. Li, J. Hamdi and M. F. Hawthorne, Bioconjugate Chem., 2006, 17, 15 CrossRef CAS PubMed; (b) E. Justus, D. Awad, M. Hohnholt, T. Schaffran, K. Edwards, G. Karlsson, L. Damian and D. Gabel, Bioconjugate Chem., 2007, 18, 1287 CrossRef CAS PubMed; (c) X. Pan, G. Wu, W. Yang, R. F. Barth, W. Tjarks and R. J. Lee, Bioconjugate Chem., 2007, 18, 101 CrossRef CAS PubMed.
- P. J. Kueffer, C. A. Maitz, A. A. Khan, S. A. Schuster, N. I. Shlyakhtina, S. S. Jalisatgi, J. D. Brockman, D. W. Nigg and M. F. Hawthorne, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 6512 CrossRef CAS PubMed.
- H. Koganei, M. Ueno, S. Tachikawa, L. Tasaki, H. S. Ban, M. Suzuki, K. Shiraishi, K. Kawano, M. Yokoyama, Y. Maitani, K. Ono and H. Nakamura, Bioconjugate Chem., 2013, 24, 124 CrossRef CAS PubMed.
- T. Peymann, C. B. Knobler and M. F. Hawthorne, Inorg. Chem., 2000, 39, 1163 CrossRef CAS.
- W. R. Hertler and M. S. Raasch, J. Am. Chem. Soc., 1964, 86, 3661 CrossRef CAS.
- (a) D. Gabel, D. Awad, T. Schaffran, D. Radovan, D. Daraban, L. Damian, M. Winterhalter, G. Karlsson and K. Edwards, ChemMedChem, 2007, 2, 51 CrossRef CAS PubMed; (b) D. Awad, L. Damian, M. Winterhalter, G. Karlsson, K. Edwards and D. Gabel, Chem. Phys. Lipids, 2009, 157, 78 CrossRef CAS PubMed.
- All protocols were approved by the Institutional Animal Care and Use Committee of Gakushuin University.
- Conventionally, a preinjection of liposome at a high dose was given for an achievement of targeting through saturation of liver's scavenging capacity (see; Y. Y. Kao and R. L. Juliano, Biochim. Biophys. Acta, 1981, 677, 453 CrossRef CAS ). Such a high liposome dose may cause possible liver toxicity, since the liver's normal scavenging function is impaired. Indeed, an important character of an approved liposomal formulation (doxil) encapsulating an anticancer drug doxorubicin is the very high concentration of the encapsulated drug (also see; P. G. Tardi, N. L. Boman and P. R. Cullis, J. Drug Targeting, 1996, 4, 129 CrossRef PubMed ).
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and data. See DOI: 10.1039/c4cc04344h |
| This journal is © The Royal Society of Chemistry 2014 |

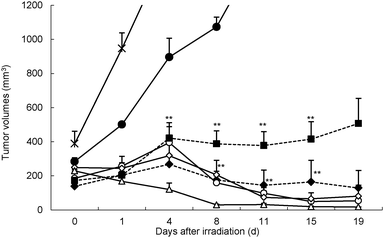
![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) , spd-BSH: 30 mg [10B] kg−1; ◆, spd-BSH: 15 mg [10B] kg−1; ◇, spd-10B12H11NH3: 15 mg [10B] kg−1) for 50 min (1.3–2.2 × 1012 neutrons per cm2). ×, cold control; ●, hot control. Mice were sacrificed when their tumor volumes reached ∼3000 mm3.
, spd-BSH: 30 mg [10B] kg−1; ◆, spd-BSH: 15 mg [10B] kg−1; ◇, spd-10B12H11NH3: 15 mg [10B] kg−1) for 50 min (1.3–2.2 × 1012 neutrons per cm2). ×, cold control; ●, hot control. Mice were sacrificed when their tumor volumes reached ∼3000 mm3.