Disulfide reshuffling triggers the release of a thiol-free anti-HIV agent to make up fast-acting, potent macromolecular prodrugs†
Abstract
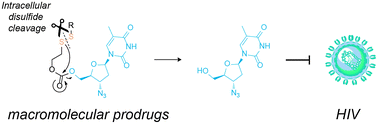
The release of azidothymidine from macromolecular prodrugs was designed to respond to the intracellular disulfide reshuffling. This drug has no thiol groups, and a response to this trigger was engineered using a self-immolative linker. The resulting formulations were fast-acting, efficacious, and highly potent with regards to suppressing the infectivity of the virus.

 Please wait while we load your content...
Please wait while we load your content...