Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly cis-selective and lead-free hydrogenation of 2-hexyne by a supported Pd catalyst with an ionic-liquid layer†
Frederick
Schwab
,
Natascha
Weidler
,
Martin
Lucas
and
Peter
Claus
*
Technische Universität Darmstadt, Ernst-Berl-Institut, Technische Chemie II, Alarich-Weiss-Straße 8, 64287 Darmstadt, Germany. E-mail: claus@ct.chemie.tu-darmstadt.de; Fax: +49 6151 16 4788; Tel: +49 6151 16 5369
First published on 22nd July 2014
Abstract
A simple Pd/SiO2 catalyst which was modified with the ionic liquid [BMPL][DCA] gave an excellent yield of 88% towards cis-2-hexene in the stereoselective hydrogenation of 2-hexyne. The catalyst outperforms, even at full conversion, the commonly used lead-poisoned, toxic Lindlar catalyst and supported colloidal-based Pd as well.
The stereoselective hydrogenation of the triple bond of internal alkynes is of great industrial interest for the production of fine chemicals or pharmaceuticals. Beside the challenge to control the stereoselectivity, also over-hydrogenation of the desired cis-alkene and its isomerization must be avoided. Therefore, in the hydrogenation of 2-hexyne a suitable catalyst has to (i) produce cis-2-hexene with high yield, (ii) prevent the consecutive hydrogenation to hexane and the isomerization to trans-2-hexene (Scheme 1). The favored intermediate cis-2-hexene could also isomerize to 1- or 3-hexene where also cis- or trans-isomers are possible.
The state-of-the-art catalyst system for the stereoselective hydrogenation of alkynes to cis-alkenes is the Lindlar catalyst containing a quinoline treated, CaCO3 supported Pd catalyst which is poisoned by toxic Pb.1
Several approaches have been made to avoid the toxic lead compound in accordance to the 12 principles of Green Chemistry.2 The modification of catalysts with other second metals like Ni or Bi reached a similar performance of the catalyst in the hydrogenation of hexynes. An increased activity compared to the Lindlar system was the main advantage.3 The influence of the preparation parameters of immobilized PdOxHy colloids on catalytic activity was investigated by Klasovsky et al.4 The optimized catalyst showed both a superior activity and selectivity to cis-2-hexene.
The usage of Pd nanoparticles in ionic liquids (IL‡) as catalysts showed a great impact on the hydrogenation of hexynes. With these new catalysts the same performance as the Lindlar system can be obtained, in some cases with even higher selectivities. However, nanoparticles stabilized with ionic liquids often require biphasic operation mode for alkyne semi-hydrogenation, additional solvents and modifiers.5
Another promising modification of heterogeneous catalysts with ionic liquids is the so-called SCILL‡ (Solid Catalyst with an Ionic Liquid Layer) concept successfully applied for hydrogenations.6,7 The concept takes into account the variability of their physico-chemical properties, and herein, the impact of the IL can be considered two-fold: (i) the surface concentration of the educt/intermediate/product of a consecutive reaction is dependent on their solubility in the IL which was described as a “physical solvent effect”, (ii) the catalytic properties of supported metal particles can be influenced directly by the IL acting as ligand in a similar way how a second metal may modify the active sites of a parent metal in bimetallic catalysts (“ligand effect”).8
One example is the SCILL catalyzed citral hydrogenation to citronellal investigated by Arras et al.7 With the SCILL system 50 wt% [BMIM][DCA]-Pd/C a quantitative yield of citronellal was achieved while the neat Pd/C catalyst only reached a selectivity of 41% at full conversion. The over-hydrogenation of citronellal to dihydrocitronellal was completely suppressed by the SCILL system. The influence of the IL was described as a new ligand effect due to the DCA (dicyanamide) anion which modified the active sites directly.8
In a recent study, Herrmann et al.9 used the SCILL concept in the gas-phase selective hydrogenation of acetylene to ethylene and could enhance the selectivity towards ethylene.
In this work, the SCILL concept was applied in the stereoselective hydrogenation of 2-hexyne to cis-2-hexene for the first time. The influence of three ionic liquids as well as the effect of the amount of the IL were investigated. The best SCILL catalyst was compared to the commercial Lindlar catalyst and the NanoSelect™ system from BASF.10 Note, that the selectivity produced by different catalysts must be compared at equal conversion so that selectivity-conversion-plots are shown.
The hydrogenation of 2-hexyne catalyzed by the neat 1Pd/SiO2 followed a typical consecutive reaction because the intermediate cis-2-hexene was isomerized and further hydrogenated to the by-products shown in Scheme 1. These side reactions led to a maximum yield of cis-2-hexene of only 61% and a complete consumption at full conversion (Fig. 1). Then, this catalyst was modified with three different ionic liquids which are N-butyl-N-methyl-pyrrolidinium dicyanamide ([BMPL][DCA]), 1-butyl-3-methyl-imidazolium hexafluorophosphate ([BMIM][PF6]) and 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)amide ([BMIM][NTf2]). Their formulas are shown in Scheme S1 (ESI†). The impregnation of the catalyst with one of these ionic liquids influenced the activity of the catalyst as well as the selectivity to cis-2-hexene. [BMIM][NTf2] hardly influenced the catalyst resulting in similar hydrogenation behavior and a maximum intermediate yield of 66% because of the solubility of this IL in the organic phase leading to a rinse off of the ionic liquid during the reaction. The [BMIM][PF6]-SCILL catalyzed hydrogenation of 2-hexyne reached an almost constant cis-2-hexene selectivity of 70% and achieved a yield of 70% at full conversion. The largest improvement of the selectivity to cis-2-hexene was achieved by modification of the Pd catalyst with 15 wt% [BMPL][DCA]. The selectivity to cis-2-hexene reached more than 80% over the whole conversion range and resulted in an excellent yield of 82% at 100% conversion. Furthermore, this SCILL system largely prevented the isomerization and over-hydrogenation of the formed cis-2-hexene because no intermediate reacted anymore, even at full conversion. In consequence of this positive impact of the ionic liquid on the semi-hydrogenation of 2-hexyne, the IL [BMPL][DCA] was used for further systematic optimization of this catalyst–reaction system.
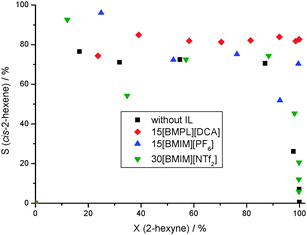 | ||
| Fig. 1 Effect of different ILs in the SCILL catalyzed hydrogenation of 2-hexyne (conditions: T = 25 °C, p(H2) = 1.04 bar, m = 350 mg Pd/SiO2-1, 5 mL 2-hexyne, 5 mL n-octane, 80 mL n-heptane). | ||
The influence of the IL amount was investigated in the range of 0 wt% < w(IL) < 50 wt% (Fig. 2). In comparison to the experiment without the IL [BMPL][DCA] the selectivity as well as the yield of cis-2-hexene was generally increased. An ionic liquid loading up to 10 wt% could not suppress the further reaction at full conversion and the desired intermediate is consumed by over-hydrogenation and isomerization. Consequently, the corresponding S–X plot, typical for consecutive reactions, was obtained in this case (Fig. 2). Further increase of the IL loading resulting in the SCILL system 30[BMPL][DCA]-1Pd/SiO2 increases tremendously the catalyst performance exhibiting an excellent yield of 88% at full 2-hexyne conversion. Surprisingly, even when the reaction approaches full conversion the selectivity towards cis-2-hexene is further increased (Fig. 2), and the corresponding concentration-time curves showed that the formation of all side-products of isomerization and over-hydrogenation is negligible (Fig. S1, ESI†). Also the catalyst with the highest amount of [BMPL][DCA] (50 wt%) is suitable for the stereoselective semi-hydrogenation (Scis-2-hexene = 80%), however, the conversion level was not quantitative (X2-hexyne = 70%). These results show that an optimum of IL loading exists where the SCILL system is beneficial with respect to high selectivity and conversion: if the IL amount is too small, the formed cis-2-hexene is consumed at very high conversion level, i.e. when the catalyst surface exhibits low alkyne concentration. In the opposite, if the IL loading is too high, tough the high selectivity is retained, the reaction does not progress at a certain degree of conversion (i.e., X2-hexyne ≪ 100%). This dependence is consistent with our observation of an optimum Pd/N ratio (nitrogen from PVP (poly(vinylpyrrolidone))) in supported (Hydr-)oxidic PdOxHy colloids used for 2-hexyne hydrogenation.4 Based on these results and taking into account our in-depth characterization of such SCILL systems8 we conclude that over-hydrogenation is suppressed by a ligand side-blocking effect due to the N-containing anion, (CN)2N−, of the ionic liquid. Moreover, in such a SCILL system also an electronic influence of the DCA anion on palladium was observed leading to a partial oxidation of the latter8 and, thus, influencing the adsorption strength of 2-hexyne/cis-2-hexene and hydrogen as well. Indeed, DCA based SCILL decreases (i) the amount of chemisorbed hydrogen suppressing the further hydrogenation and (ii) the adsorption enthalpy of hydrogen8 which indicated, compared to the non-modified Pd catalyst, a weaker hydrogen bonding making the SCILL more selective.
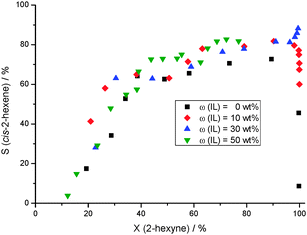 | ||
| Fig. 2 Effect of the amount of [BMPL][DCA] (conditions: T = 25 °C, p(H2) = 1.04 bar, m = 350 mg Pd/SiO2-2, 5 mL 2-hexyne, 5 mL n-octane, 80 mL n-heptane). | ||
The superior performance of this innovative SCILL system could be shown by the comparison to Pd nanoparticles in methanol described by Lee et al.5 To reach a yield of cis-2-hexene of 82%, the 8-fold higher amount of palladium was required and even the used amount of 2-hexyne was much smaller. Taking into account the different amounts of active metal and educt used in these experiments a modified space-time-yield (STY) was estimated (Table S1, ESI†). The catalyst 30[BMPL][DCA]-1Pd/SiO2 reached an excellent STY of 3.8 gprod. gPd−1 min−1 which was more, at least, than one order of magnitude higher than the Pd nanoparticles of Lee et al.
The catalyst was also compared to a commercial Lindlar and NanoSelect™ catalyst from BASF. The Lindlar catalyst is the state-of-the-art catalyst for stereoselective hydrogenation of internal alkynes as described above. The NanoSelect™ catalyst is a Lead-free nano-colloid which consists of supported Pd nanoparticles. The Pd content is 10-fold lower than that of the Lindlar system producing cis-products with high selectivity.10
The commercial Lindlar and NanoSelect™ catalysts showed a similar selectivity to cis-2-hexene almost over the whole conversion range as the SCILL 30[BMPL][DCA]-1Pd/SiO2 (Fig. 3). However, it must be noted that there is a main difference between these commercial catalysts and our SCILL system, and this most striking effect is the performance near and at 100% conversion. The modification of the Pd catalyst with the ionic liquid completely suppressed the further reaction of the intermediate, whereas the commercial systems catalyzed the hydrogenation and isomerization of cis-2-hexene which led to a decrease of the yield of the desired product for the Lindlar and NanoSelect™ catalyst of 74% and 71%, respectively (see also yield vs. time plot, Fig. S2, ESI†). In other words, at these conditions the SCILL system outperformed the commercially available catalysts for the stereoselective hydrogenation of internal alkynes reaching a yield of cis-2-hexene of 88% at full conversion in the hydrogenation of 2-hexyne.
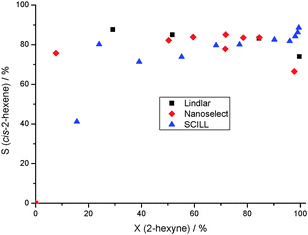 | ||
| Fig. 3 Comparison of the SCILL with industrial catalysts used as reference (conditions: T = 25 °C, p(H2) = 1.04 bar, 5 mL 2-hexyne, 5 mL n-octane, 80 mL n-heptane). | ||
In general, the high alkene selectivity is attributed to the stronger adsorption of alkynes compared to the corresponding alkenes on Pd determining the relative surface coverage of the adsorbed alkyne vs. alkene. Note, that it was recently shown in gas-phase alkyne hydrogenation that the high selectivity towards alkene is due to the formation of a Pd-carbide like phase.11 Following the thermodynamic argumentation, the similar performance of the Pd catalysts up to 95% conversion (Fig. 3) can be retraced. The usually observed sharp decrease in selectivity, when the reaction approaches full conversion, is ascribed to alkene re-adsorption followed by hydrogenation. This is prevented with the SCILL system because the alkene is less soluble in the IL than the alkyne enabling faster alkene desorption and preventing its re-adsorption. It follows that, beside the finding that the IL directly modifies the Pd sites by ligand-effects, also reactant solubility in the IL film is involved in controlling the selectivity. Taking further into account the poor solubility and slow diffusion of hydrogen in the IL we propose that also the formation of Pd bulk-dissolved (subsurface) hydrogen can be avoided by the IL layer. The presence of such kind of hydrogen would shift the selectivity towards the alkane,11i.e. then over-hydrogenation occurs.
In summary, for the first time the SCILL concept is successfully applied in the stereoselective hydrogenation of 2-hexyne to cis-2-hexene which is produced with an outstanding yield of 88%. The catalytic properties of this innovative and environmentally benign SCILL system outperform the industrially used Lindlar and NanoSelect™ catalyst even when the reaction approaches full conversion. In the next step, the SCILL system will be transformed in a shaped product for continuous hydrogenation in order to study catalyst stability with time-on-stream.
Notes and references
- H. Lindlar, Helv. Chim. Acta, 1952, 35, 446 CrossRef CAS.
- S. L. Y. Tang, R. L. Smith and M. Poliakoff, Green Chem., 2005, 7, 761 RSC.
- J. A. Anderson, J. Mellor and R. P. K. Wells, J. Catal., 2009, 261, 208 CrossRef CAS PubMed; M. J. Maccarrone, C. R. Lederhos, G. Torres, C. Betti, F. Coloma-Pascual, M. E. Quiroga and J. C. Yori, Appl. Catal., 2012, 441–442, 90 CrossRef PubMed.
- F. Klasovsky, P. Claus and D. Wolf, Top. Catal., 2009, 52, 412 CrossRef CAS.
- J. K. Lee, D. W. Kim, M. Cheong, H. Lee, B. W. Cho, H. S. Kim and D. Mukherjee, Bull. Korean Chem. Soc., 2010, 31, 2195 CrossRef CAS; R. Venkatesan, M. H. G. Prechtl, J. D. Scholten, R. P. Pezzi, G. Machado and J. Dupont, J. Mater. Chem., 2011, 21, 3030 RSC.
- U. Kernchen, B. Etzold, W. Korth and A. Jess, Chem. Eng. Technol., 2007, 30, 985 CrossRef CAS.
- J. Arras, M. Steffan, Y. Shayeghi and P. Claus, Chem. Commun., 2008, 4058 RSC; J. Arras, M. Steffan, Y. Shayeghi, D. Ruppert and P. Claus, Green Chem., 2009, 11, 716 RSC.
- J. Arras, E. Paki, C. Roth, J. Radnik, M. Lucas and P. Claus, J. Phys. Chem. C, 2010, 114, 10520 CAS.
- T. Herrmann, L. Rößmann, M. Lucas and P. Claus, Chem. Commun., 2011, 47, 12310 RSC.
- P. T. Witte, P. H. Berben, S. Boland, E. H. Boymans, D. Vogt, J. W. Geus and J. G. Donkervoort, Top. Catal., 2012, 55, 505 CrossRef CAS.
- D. Teschner, J. Borsodi, A. Wootsch, Z. Révay, M. Hävecker, A. Knop-Gericke, S. D. Jackson and R. Schlögl, Science, 2008, 320, 86 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cc04183f |
| ‡ Abbreviations: IL = ionic liquid, SCILL = solid catalyst with an ionic liquid layer, [BMIM][PF6] = 1-butyl-3-methyl-imidazolium hexafluorophosphate, [BMIM][NTf2] = 1-butyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)amide, [BMPL][DCA] = N-butyl-N-methyl-pyrrolidinium dicyanamide, 30[IL]-1Pd/SiO2 = the catalyst 1 wt% Pd supported by silica was impregnated with 30 wt% of the ionic liquid.Catalyst preparation: the SCILL catalysts were prepared via incipient-wetness impregnation to give a 1 wt% Pd/SiO2. After drying the support material (2 h, 200 °C, charge 1, or 130 °C, charge 2), a certain amount of Pd(AcO)2 was dissolved in acetone and impregnated on the silica. The charges of the parent catalyst, denoted by Pd/SiO2-1 and Pd/SiO2-2, were dried (25 °C, 15 h) and reduced (100 °C, 100 mL min−1 H2, 1 h). Then, the catalysts were modified by different ILs via incipient-wetness impregnation as well (solvent: acetone). The resulting SCILL catalysts were dried (25 °C, 15 h). Catalyst testing: the hydrogenation experiments were performed in a batch reactor (300 mL, Parr Instruments) at 1.04 bar H2, 25 °C and a stirring rate of 1000 min−1. An educt mixture of 5 mL 2-hexyne, 5 mL n-octane (internal GC standard) and 80 mL n-heptane as well as 350 mg of the catalyst were used in each experiment. |
| This journal is © The Royal Society of Chemistry 2014 |