Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Terbium(III)-cholate functionalized vesicles as luminescent indicators for the enzymatic conversion of dihydroxynaphthalene diesters†
Stefan
Balk
a,
Uday
Maitra
*b and
Burkhard
König
*a
aFaculty of Chemistry and Pharmacy, University of Regensburg, 93040 Regensburg, Germany. E-mail: burkhard.koenig@ur.de; Fax: +49 943 1717; Tel: +49 943 4576
bDepartment of Organic Chemistry, Indian Institute of Science, Bangalore, India. E-mail: maitra@orgchem.iisc.ernet.in; Fax: +91-80-2360-0529; Tel: +91-80-2360-1968
First published on 3rd June 2014
Abstract
The phosphorescence intensity of unilamellar DOPC vesicles with embedded Tb3+-cholate complexes depends on the concentration of dihydroxynaphthalene (DHN) as sensitizer in solution. This was used to monitor the enzymatic conversion of DHN esters or DHN glucosides by enzymes in aqueous buffered solution.
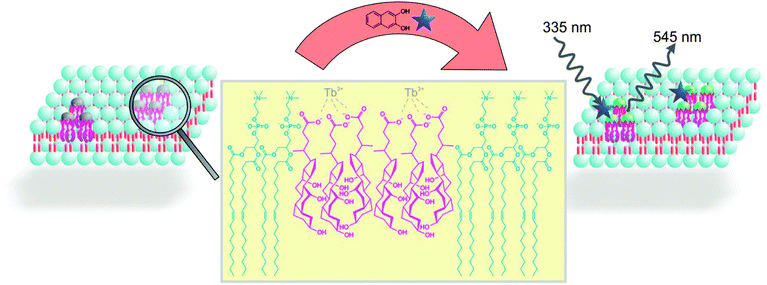
Cholic acids are known to aggregate in the presence of trivalent lanthanide ions resulting in three dimensional networks of hydrogels.1 Such gels have found applications as optical materials,1a,b,2 in the preparation of nanoparticles,1c,3 as confined reaction media4 and in the detection of analytes.5 The lanthanide luminescence of hydrogels prepared from sodium cholate and terbium(III) salt is sensitized by 2,3-dihydroxynaphthalene (DHN). Only the dihydroxy compound coordinates to the Tb3+ ion and acts as sensitizer, but not DHN ester or acetal derivatives.1a This observation has been used to monitor the enzymatic conversion of carboxy esters or the monoglucoside of DHN by changes in the phosphorescence intensity avoiding interference with background fluorescence.6,7 The luminescent gel indicator is readily prepared by self-assembly of all components in aqueous buffer, but the three dimensional gel limits the diffusion and the enzymes have to be added during gel preparation. Therefore we transferred this detection mechanism from hydrogels to the membrane of small unilamellar vesicles (Scheme 1). Functionalized lipid bilayers have been previously used in enzyme assays8 or as luminescent indicators.9
A vesicular solution of 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC, 5 mM) in HEPES buffer (25 mM, pH 7.4), was prepared by extrusion in the presence of a submicellar concentration‡ of cholic acid (0.75 mM). The cholic acid will phase separate in the DOPC membrane and added TbCl3·6H2O (0.25 mM) coordinates to the membrane embedded bile salts. Alternatively, a post functionalization of DOPC vesicles by bile salts and Tb(III) is possible, when the components are added to buffered DOPC vesicle solutions. We assume the formation of membrane anchored terbium(III)-complex domains. The resulting vesicle solutions are homogeneous, stable and monodisperse.
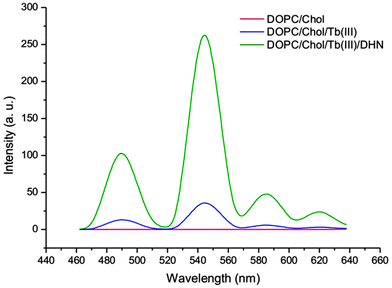
Next, DHN (12.5 μM) was added to the aqueous vesicle solution Vs1 (C(DOPC) = 5 mM, C(Chol) = 0.75 mM). Excitation of the mixture at 335 nm gave a significant terbium luminescence with an emission maximum at 545 nm (Scheme 2). Previous studies have shown that the DHN sensitization of the lanthanide emission requires a rigid gel matrix.1a,5a The strong increase of terbium luminescence in the vesicle membrane therefore indicates that the Tb3+-cholate patches might have gel like properties.
The presence of cholic acid, DHN and terbium(III) salts as membrane additives is essential to observe the lanthanide emission (Scheme 2), which was confirmed by different DOPC vesicle solutions (Vs2: C(DOPC) = 5 mM, Vs1: C(DOPC) = 5 mM, C(Chol) = 0.75 mM, Vs3: C(DOPC) = 5 mM, C(Chol) = 0.75 mM, C(Tb3+) = 0.25 mM, C(DHN) = 12.5 μM) and post functionalized DOPC vesicles (Vs3p: C(DOPC) = 5 mM, C(Chol) = 0.75 mM, C(Tb3+) = 0.25 mM, C(DHN) = 12.5 μM). Dynamic light scattering (DLS) confirmed a monodisperse narrow size distribution around 110 nm for all samples. In contrast, solutions of cholate monomers (0.75 mM) in HEPES buffer (25 mM, pH 7.4) or CHCl3 showed a broad size distribution and polydispersity (see ESI† for data). The embedding of the luminescent cholic acid–terbium complexes in the vesicle membrane was confirmed by fluorescence anisotropy: the terbium complex, excited by DHN (12.5 μM) at 335 nm, and bound to cholic acid doped vesicles Vs4 (C(DOPC) = 5 mM, C(Chol) = 0.75 mM, C(Tb3+) = 0.25 mM) showed a fluorescence anisotropy, detected at 550 nm, which is about 8 times higher than for terbium in aqueous cholic acid solution (C(Chol) = 0.75 mM, C(Tb3+) = 0.25 mM, C(DHN) = 12.5 μM). Higher anisotropy values detected at 380 nm of DHN (12.5 μM) in the presence of Vs4 compared to aqueous cholate solution indicated the coordination of DHN to terbium(III) ions at the surface of vesicle Vs4 (see ESI† for data).
Since DHN derivatives lacking free hydroxy groups for metal coordination, do not sensitize the terbium(III) emission, reactions converting DHN derivatives into DHN can be easily monitored by the functionalized vesicles. Lipase (Candida rugosa, 2.5 U mg−1, 50 mg L−1) was added to the aqueous vesicle solution Vs1 (C(DOPC) = 5 mM, C(Chol) = 0.75 mM) with added TbCl3·6H2O (0.25 mM) and naphthalene-2,3,-diyl dihexanoate (DHNdhn, 12.5 μM) or naphthalene-2,3-diyl diacetate (DHNdac). The enzymatic ester cleavage of DHNdhn into DHN leads to an increase of the vesicle luminescence intensity tracing the reaction (Schemes 3 and 4). 3-Hydroxynaphthalene-2-yl-β-glucoside (DHNglu) was likewise used to monitor the enzymatic activity of β-glucosidase (from almonds, 6.5 U mg−1, 50 mg L−1).
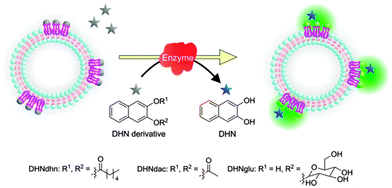 | ||
| Scheme 3 Lipase conversion of naphthalene-2,3-dihydroxyesters (12.5 μM) into DHN leads to an increase of the terbium luminescence intensity. | ||
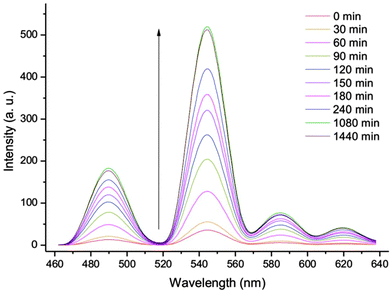 | ||
| Scheme 4 Luminescence intensity of Vs4 (C(DOPC) = 5 mM, C(Chol) = 0.75 mM, C(Tb3+) = 0.25 mM) is increasing in the presence of DHNdhn (12.5 μM) and lipase (50 mg L−1) over 24 h. | ||
To confirm that the emission intensity change during the reaction correlates with the amount of produced DHN, we monitored the enzymatic conversion by HPLC in the absence of vesicles. The initial rate constants for the esterase activity of lipase derived from the emission intensity increase or the HPLC analysis of produced DHN were comparable with 1.3 × 10−8 and 1.9 × 10−8 mmol min−1, respectively, (see ESI† for data).§ A detection limit of 0.5 mg L−1 for lipase activity was determined for the assay using a 24 h incubation time, which significantly improved compared to the previously used hydrogels that required 900 mg L−1.5a
In conclusion, we have embedded terbium-cholate aggregates into the membrane of 100 nm unilamellar DOPC vesicles. Dihydroxynaphthalene coordinates to the complexes at the membrane–water interface and sensitizes the terbium phosphorescence. As the concentration of free dihydroxynaphthalene in the aqueous solution correlates with the terbium phosphorescence intensity, enzymatic reactions of dihydroxynaphthalene esters and glycosides can be monitored in buffered aqueous solution. The phosphorescent vesicular indicator is easily prepared by self-assembly and many DHN derivatives as pro-sensitizers can be envisaged that are suitable substrates for a variety of enzymes. The detection principle may therefore find application as a facile luminescent on-line monitoring of enzymatic activity.
SB thanks the INDIGO network of the German Academic Exchange Service (DAAD) for travel support.
Notes and references
- (a) S. Banerjee, R. Kandanelli, S. Bhowmik and U. Maitra, Soft Matter, 2011, 7, 8207–8215 RSC; (b) S. Bhowmik, S. Banerjee and U. Maitra, Chem. Commun., 2010, 46, 8642–8644 RSC; (c) A. Chakrabarty, U. Maitra and A. D. Das, J. Mater. Chem., 2012, 22, 18268–18274 RSC; (d) H. Svobodová, V. Noponen, E. Kolehmainen and E. Sievänen, RSC Adv., 2012, 2, 4985–5007 RSC; (e) For an X-ray structure analysis of lanthanide - DHN complex, see: D. I. Alexandropoulos, A. Fournet, L. Cunha-Silva, A. M. Mowson, V. Bekiari, G. Christou and T. C. Stamatatos, Inorg. Chem., 2014 DOI:10.1021/ic500806n.
- (a) Y. Qiao, Y. Lin, S. Zhang and J. Huang, Chem. – Eur. J., 2011, 17, 5180–5187 CrossRef CAS PubMed; (b) X. Ma, D. Yu, N. Tang and J. Wu, Dalton Trans., 2014 10.1039/c4dt00110a.
- Y. Qiao, H. Chen, Y. Lin, Z. Yang, X. Cheng and J. Huang, J. Phys. Chem. C, 2011, 115, 7323–7330 CAS.
- (a) S. Bhat and U. Maitra, Molecules, 2007, 12, 2181–2189 CrossRef CAS; (b) J. Bachl, A. Hohenleutner, B. B. Dhar, C. Cativiela, U. Maitra, B. Konig and D. D. Diaz, J. Mater. Chem. A, 2013, 1, 4577–4588 RSC.
- (a) S. Bhowmik and U. Maitra, Chem. Commun., 2012, 48, 4624–4626 RSC; (b) S. Mizukami, K. Tonai, M. Kaneko and K. Kikuchi, J. Am. Chem. Soc., 2008, 130, 14376–14377 CrossRef CAS PubMed; (c) T. Terai, K. Kikuchi, Y. Urano, H. Kojima and T. Nagano, Chem. Commun., 2012, 48, 2234–2236 RSC; (d) T. Terai, H. Ito, K. Kikuchi and T. Nagano, Chem. – Eur. J., 2012, 18, 7377–7381 CrossRef CAS PubMed.
- (a) T. Steinkamp, F. Schweppe, B. Krebs and U. Karst, Analyst, 2003, 128, 29–31 RSC; (b) K.-H. Leung, H.-Z. He, V. P.-Y. Ma, H.-J. Zhong, D. S.-H. Chan, J. Zhou, J.-L. Mergny, C.-H. Leung and D.-L. Ma, Chem. Commun., 2013, 49, 5630–5632 RSC; (c) B. K. McMahon and T. Gunnlaugsson, J. Am. Chem. Soc., 2012, 134, 10725–10728 CrossRef CAS PubMed; (d) U. Reddy G, P. Das, S. Saha, M. Baidya, S. K. Ghosh and A. Das, Chem. Commun., 2013, 49, 255–257 RSC; (e) J. Hu, G. Zhang and S. Liu, Chem. Soc. Rev., 2012, 41, 5933–5949 RSC.
- Reviews on lanthanide probes for the determination of enzymatic activity, see: (a) C. M. Spangler, C. Spangler and M. Schäerling, Ann. N. Y. Acad. Sci., 2008, 1130, 138–148 CrossRef CAS PubMed; (b) E. F. Gudgin Dickson, A. Pollak and E. P. Diamandis, J. Photochem. Photobiol., B, 1995, 27, 3–19 CrossRef CAS; (c) R. A. Evangelista, A. Pollak and E. F. Gudgin Templeton, Anal. Biochem., 1991, 197, 213–224 CrossRef CAS.
- (a) G. Das, P. Talukdar and S. Matile, Science, 2002, 298, 1600–1602 CrossRef CAS PubMed; (b) T. Takeuchi and S. Matile, Chem. Commun., 2013, 49, 19–29 RSC; (c) P. Walde and S. Ichikawa, Biomol. Eng., 2001, 18, 143–177 CrossRef CAS.
- (a) B. Gruber and B. König, Chem. – Eur. J., 2013, 19, 438–448 CrossRef CAS PubMed; (b) B. Gruber, S. Balk, S. Stadlbauer and B. König, Angew. Chem., Int. Ed., 2012, 51, 10060–10063 CrossRef CAS PubMed; (c) S. Banerjee and B. König, J. Am. Chem. Soc., 2013, 135, 2967–2970 CrossRef CAS PubMed; (d) B. Gruber, S. Stadlbauer, A. Späth, S. Weiss, M. Kalinina and B. König, Angew. Chem., Int. Ed., 2010, 49, 7125–7128 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cc03724c |
| ‡ Critical micellar concentration (cmc) for sodium cholate is 9–14 mM. |
| § Non-enzymatic spontaneous hydrolysis of DHN esters over 24 h is negligible. |
| This journal is © The Royal Society of Chemistry 2014 |