Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A non-aqueous procedure to synthesize amino group bearing nanostructured organic–inorganic hybrid materials†
M.
Göring
a,
A.
Seifert
a,
K.
Schreiter
a,
P.
Müller
b and
S.
Spange
*a
aDepartment of Polymer Chemistry, Institute of Chemistry, Technische Universität Chemnitz, Strasse der Nationen 62, D-09111 Chemnitz, Germany. E-mail: stefan.spange@chemie.tu-chemnitz.de
bBASF SE, Carl-Bosch Straße 38, D-67056 Ludwigshafen, Germany
First published on 7th July 2014
Abstract
Amino-functionalized organic–inorganic hybrid materials with a narrow distributed nanostructure of 2–4 nm in size were obtained by means of a template-free and non-aqueous procedure. Simultaneous twin polymerization of novel amino group containing twin monomers with 2,2′-spirobi[4H-1,3,2-benzodioxasiline] has been applied for this purpose. The amino groups of the organic–inorganic hybrid material are useful for post derivatization.
Organic–inorganic hybrid materials, which contain amino groups, are of great importance for a variety of applications such as supports for catalysts, adsorption of metal ions, or as key precursors for post derivatization.1,2 It is an important task to obtain nanostructured materials with accessible amino groups due to their great potential for post-functionalization reactions with electrophilic reagents.3–5 Amino functionalized silica materials have been successfully used for CO2,6 water7,8 or dye absorption7 and catalysis of Knoevenagel condensation reactions9,10 and the synthesis of nitrostyrenes.11 Furthermore, primary and secondary amino groups can be modified in many ways to introduce carboxyl groups from cyclic anhydrides or imine groups from aldehydes.
These materials can be synthesized by different strategies which use amino-functionalized reagents.1,2,8,12,13 Suitable reagents are 3-aminopropylalkoxysilanes or amino group bearing water soluble polymers such as polyvinylamine.13 The synthetic procedure employing these reagents is based on water as a co-reagent or a solvent. Therefore, co-reagents which contain hydrolytically sensitive groups such as imines or isocyanates cannot by applied when water based procedures are used. To avoid these peculiarities, a synthetic method is needed which does not require water as a solvent or a reagent.
Twin-polymerization can be carried out either in organic solvents or even in the melt without the use of any solvent.14,15 This advantage overcomes all problems resulting from water chemistry. Furthermore, amino groups undergo acid–base reactions with water, and ammonium ions are formed which can have a negative effect on post-reactions of amino groups.
In this communication a specific application of twin-polymerization is presented which uses a non-aqueous procedure involving various amino group bearing twin monomers to produce nanostructured organic–inorganic hybrid materials related to amino group containing sol–gel materials.
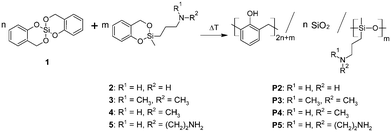
During the last six years the so called twin polymerization has been developed to fabricate organic–inorganic hybrid materials by solely one procedure.14–18 The principle of twin-polymerization is based on specific twin monomers (TMs), which contain two covalently bonded polymerizable monomeric fragments, i.e. A and B for each polymer. The formation of the two polymers from the twin monomers during polymerization is mechanistically coupled.17 Thus, polymer –(A)n– can only be formed when polymer –(B)n– is also formed. This is a crucial difference in the polymerization behavior from that of hetero-bifunctional monomers whose two different polymer strands are mechanistically independent of each other during the polymerization procedure.4,5,13 Simultaneous twin polymerization (STP) of two different twin monomers in one process can yield up to four different polymers (see also Fig. S1 ESI†).18
A benefit of this type of STP is that the formation of polymer –(A)n– mediates the covalent connection of fragments B and C during polymerization within the organic–inorganic hybrid material.
The synthetic procedure for the amino group containing TM (2–5) starts from salicylic alcohol and 3-amino-n-propyldimethoxy-methylsilanes. The transesterification reaction was catalyzed by tetra-n-butylammonium fluoride (TBAF) (see Fig. 2). Details of the synthetic procedures are given in the ESI.†
Objective of this basic study is the synthetic feasibility of new types of TMs and to study their polymerization by STP to produce hybrid materials bearing accessible amino groups. The STP of 2,2′-spirobi[4H-1,3,2-benzodioxasiline] (1) with amino group functionalized twin monomers has been studied.
Pure 1 undergoes twin polymerization by either an acid- or a base-catalyzed reaction in melt or in solution.19,20 However, it is also possible to polymerize 1 without the use of any catalyst at 230 °C.19
Advantageously, the amino functionalized monomers (2–5) used for the STP can serve as both a component and a basic catalyst. This is an elegant way of synthesis, because impurities resulting from the use of additional catalysts can be avoided. Thus, monomer 1 was simultaneously polymerized with 2, 3, 4 or 5 at a much lower temperature (120 °C) using a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
According to the conceptual idea of STP, an organic–inorganic hybrid material is composed of phenolic resin, silica and oligo(3-amino-n-propyl)methylsiloxane (OAMS) (Fig. 1).
 | ||
| Fig. 1 Procedure for thermally induced STP of twin monomer 1 with 2, 3, 4 and 5, respectively, and appellation of the resulting hybrid materials. | ||
It is apparent from silicon chemistry that silica and OAMS moieties can undergo Si–O–Si bond formation to form a class II hybrid structure among the phenolic resin/SiO2/OAMS hybrid compound.21–23 This feature can be readily evidenced by solid state 29Si- and 13C-NMR spectroscopy shown in Fig. 2 and the ESI.†
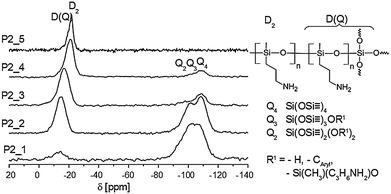
Altogether, the 29Si-CP-MAS-NMR spectra of hybrid materials (P2–P5) show the expected Q- and D-signals originating from monomer 1 and the accordant amino functionalized monomers (2–5). In each case Q4 (−110 ppm) is the most intense signal in the silica region of the spectrum, although the cross polarization technique used overrates Q2 (−90 ppm) and Q3 (−100 ppm) signals due to polarization transfer from 1H to 29Si. This result indicates that the silica network is highly condensed. The same applies to the OAMS part, as no signals from the monomer are detectable. Instead, a rather broad signal at −17.6 ppm is found, which can be assigned as D(Q).24 Parts of OAMS are covalently bonded to silica and form a copolymer, related to Co-STP as shown in Fig. 1, ESI.† The broad line widths of the D(Q)-signals are typical for a high dispersion of chemical shifts and a reduced flexibility of the OAMS moiety. The formation of phenolic resin and of OAMS can be clearly evidenced by means of 13C-CP-MAS-NMR spectroscopy. The signal-structure assignments are depicted in Fig. 2. The formation of o,o′- and o,p′-linked phenolic resin structures could be evidenced by the signal pattern in the aromatic carbon region (signals 3 and 4, Fig. 2) and a broad signal of the bridging methylene groups (signal 5). An intense signal of methyl groups of OAMS arises at 0 ppm. The other carbon atoms of the alkyl chains of OAMS are found in the expected regions (10–50 ppm). The weak signal at 62 ppm originates from unreacted Si–O–CH2 groups of the monomers (1: 66.3 ppm; 2, 3, 4 and 5: ∼63 ppm) or benzyl alcohol (–CH2–OH: 64.7 ppm). This is in agreement with the results of the 29Si-NMR (Q3), and post-reactions above 120 °C were detected by differential scanning calorimetry (DSC) measurements (Fig. S5 ESI†). Soxhlet extraction with dichloromethane (DCM) showed extractable fractions which are attributed to low molecular weight products (see ESI†). A high degree of polymerization in the case of step growth polymerizations is only achieved with nearly complete conversion of functional groups. Thus, composites of monomers 1 and 2 were synthesized at higher temperatures (180 °C; monomer 2 solely polymerizes at 220 °C) and in different ratios to P2_1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 = 95
2 = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), P2_2 (85
05), P2_2 (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15), P2_3 (50
15), P2_3 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), P2_4 (15
50), P2_4 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) and P2_5 (0
85) and P2_5 (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) (experimental details are given in the ESI†). The higher polymerization temperature reduces the extractable content of the resulting hybrid material significantly from 43 wt% (P2) to 6 wt% (P2_3), see ESI.† DSC-measurements show no more post-reactions upon thermal treatment of the materials (see ESI†). The molecular structure monitored by means of 13C- and 29Si-CP-MAS-NMR spectroscopy is not altered by the increased temperature (P2 Fig. S2 vs.P2_3 Fig. S3 ESI†).
100) (experimental details are given in the ESI†). The higher polymerization temperature reduces the extractable content of the resulting hybrid material significantly from 43 wt% (P2) to 6 wt% (P2_3), see ESI.† DSC-measurements show no more post-reactions upon thermal treatment of the materials (see ESI†). The molecular structure monitored by means of 13C- and 29Si-CP-MAS-NMR spectroscopy is not altered by the increased temperature (P2 Fig. S2 vs.P2_3 Fig. S3 ESI†).
The solid state NMR spectra again evidence the formation of the phenolic resin, silica and OAMS (Fig. S3 ESI†). The different monomer ratios are reflected in the NMR spectra and the signal intensities of phenolic resin/OAMS change accordingly. The 13C-NMR spectra show no differences in the chemical shifts. An increasing content of monomer 2 also leads to more intense D-signals as shown in the 29Si-NMR spectra (Fig. 3). A low ratio of monomer 2 results in D(Q) species. The corresponding signal arises at −17 ppm. Because of the formation of the silica-OAMS copolymer, the extractable content of sample P2_1 (0.5 wt%) is low. Longer chains (or rings) of OAMS are formed upon increasing content of monomer 2. The signal is shifted to a higher field (−21 ppm) related to D2 structures.24 Also a larger quantity of OAMS could be extracted (P2_4: 31.2 wt%) because less covalent bonds were formed between the silica network and OAMS. Due to the absence of monomer 1 the material P2_5 only shows D- without any Q signals. The intensities of the Q signals of P2_1 to P2_4 are affected by the monomer ratio used. A reduction of monomer 2 leads to increased Q3 signals because the concentration of the basic catalyst (2) is decreased.
High angle annular dark field (HAADF) scanning transmission electronmicroscopy (STEM) images of selected hybrid materials show domain sizes of 2–4 nm (Fig. 4a). This is much lower than comparable phenolic resin/silica composites synthesized by a sol–gel process.25,26 The materials are transparent and no macroscopic agglomeration could be observed in all cases (Fig. 4b and ESI†).
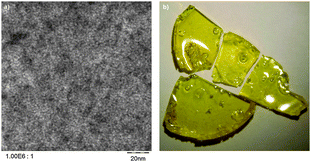 | ||
| Fig. 4 (a) HAADF-STEM image of the composite material P2 showing domains of 2–4 nm in size. (b) Macroscopic picture of the transparent, monolithic composite material. | ||
Thermogravimetric analysis (TGA) of the organic–inorganic hybrid materials shows a slight weight loss of 2.11 wt% (P2_1) to 8.66 wt% (P2_5) between 30 and 200 °C. Further decomposition of the hybrid material is comparable to that of aminopropyl-modified hybrid materials known from the literature.12
In order to check the accessibility of the amino groups in the hybrid materials after STP, the reaction with different aldehydes was studied (Fig. 5). After washing and drying procedures, the formation of the Schiff base was examined by infrared- (IR) and ultraviolet-visible (UV/Vis) spectroscopy. The treated materials show additional IR-bands at 2230, 1643 and 696 cm−1 belonging to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibrations and at 1346 cm−1 assigned to the N–O vibration of the nitro group (see ESI†). The conversion of the amino groups within the hybrid material was 14.4–66.7% determined by quantitative elemental analysis of the N-content with respect to 100% theoretical conversion (see ESI†).
N vibrations and at 1346 cm−1 assigned to the N–O vibration of the nitro group (see ESI†). The conversion of the amino groups within the hybrid material was 14.4–66.7% determined by quantitative elemental analysis of the N-content with respect to 100% theoretical conversion (see ESI†).
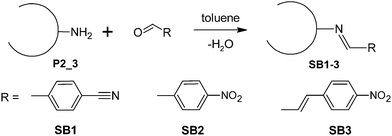 | ||
| Fig. 5 Reaction of surface amino groups with p-cyanobenzaldehyde, p-nitrobenzaldehyde and p-nitro-cinnamaldehyde. | ||
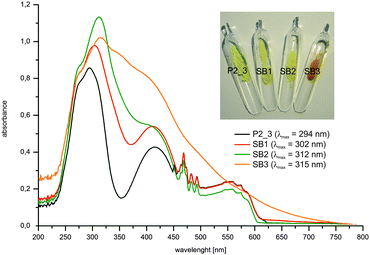
The functionalized hybrid materials SB1-3 obtained have yellow to orange color shades. UV/Vis absorption spectra of the untreated composite P2_3 and SB1-3 have been recorded by means of diffuse reflectance UV/Vis spectroscopy. The UV/Vis absorption bands are non-symmetric and show several UV/Vis absorption maxima. A bathochromic shift of the UV/Vis absorption band can be observed for Schiff bases SB1-3 in comparison to the untreated composite material P2_3 (λmax = 294 nm) (Δṽ (P2_3 → SB3) = 2268 cm−1), which confirms the accessibility of the amino groups and the formation of Schiff bases (Fig. 6). In addition, the UV/Vis absorption maximum of the Schiff bases shifts bathochromically from 302 nm (SB1) to 312 nm (SB2) and 315 nm (SB3). This is caused by an increase in the strength of the push–pull π-electron system with the increasing accepting capacity of the corresponding substituent (CN → NO2).
We can conclude that monolithic nanostructured (phenolic resin/aminosilane/SiO2) organic–inorganic hybrid materials obtained from 1 (2,2′-spirobi[4H-1,3,2-benzodioxazoiline]) and various N-substituted 2-(3-amino-n-propyl)-2-methyl-4H-1,3,2-benzodioxazoiline derivatives (2–5) can be produced in the melt by self-induced simultaneous twin polymerization at 180 °C. The molecular structures of the organic–inorganic hybrid materials have been evidenced by solid state 13C- and 29Si-NMR spectroscopy. Intactness of the primary amino groups has been proved by reactions with aldehydes to achieve azomethine moieties (SB1–3), which can be detected readily by UV/Vis spectroscopy.
Application of this specific type of twin monomer combination in heat-induced coating, particle fabrication or other purposes will be demonstrated soon.
This work was performed within the Federal Cluster of Excellence EXC 1075 “MERGE Technologies for Multifunctional Lightweight Structures” and DFG SP 392/34-1, supported by the German Research Foundation (DFG). Financial support is gratefully acknowledged.
Notes and references
- F. Hoffmann, M. Cornelius, J. Morell and M. Fröba, Angew. Chem., 2006, 118, 3290–3328 CrossRef.
- J. E. Lofgreen and G. A. Ozin, Chem. Soc. Rev., 2014, 43, 911–933 RSC.
- L. Ni, N. Moreau, A. Chemtob and C. Croutxé-Barghorn, J. Sol-Gel Sci. Technol., 2012, 64, 500–509 CrossRef CAS.
- M. Sakeye and J.-H. Smått, Langmuir, 2012, 28, 16941–16950 CrossRef CAS PubMed.
- R. Voss, A. Thomas, M. Antonietti and G. A. Ozin, J. Mater. Chem., 2005, 15, 4010–4014 RSC.
- S. Araki, H. Doi, Y. Sano, S. Tanaka and Y. Miyake, J. Colloid Interface Sci., 2009, 339, 382–389 CrossRef CAS PubMed.
- Z. Wu, H. Xiang, T. Kim, M.-S. Chun and K. Lee, J. Colloid Interface Sci., 2006, 304, 119–124 CrossRef CAS PubMed.
- G. G. Paradis, R. Kreiter, M. M. A. van Tuel, A. Nijmeijer and J. F. Vente, J. Mater. Chem., 2012, 22, 7258–7264 RSC.
- D. J. Macquarrie and D. B. Jackson, Chem. Commun., 1997, 1781–1782 RSC.
- B. M. Choudary, M. L. Kantam, P. Sreekanth, T. Bandopadhyay, F. Figueras and A. Tuel, J. Mol. Catal. A: Chem., 1999, 142, 361–365 CrossRef CAS.
- G. Sartori, J. Catal., 2004, 222, 410–418 CrossRef CAS PubMed.
- R. Brambilla, J. Poisson, C. Radtke, M. S. L. Miranda, M. B. Cardoso, I. S. Butler and J. H. Z. dos Santos, J. Sol-Gel Sci. Technol., 2011, 59, 135–144 CrossRef CAS PubMed.
- I. Voigt, F. Simon, K. Estel and S. Spange, Langmuir, 2001, 17, 3080–3086 CrossRef CAS.
- S. Grund, P. Kempe, G. Baumann, A. Seifert and S. Spange, Angew. Chem., Int. Ed., 2007, 46, 628–632 CrossRef CAS PubMed.
- S. Spange and S. Grund, Adv. Mater., 2009, 21, 2111–2116 CrossRef CAS.
- P. Kempe, T. Löschner, A. A. Auer, A. Seifert, G. Cox and S. Spange, Chem. – Eur. J., 2014, 20, 8040–8053 CrossRef CAS PubMed.
- A. A. Auer, A. Richter, A. V. Berezkin, D. V. Guseva and S. Spange, Macromol. Theory Simul., 2012, 21, 615–628 CrossRef CAS.
- T. Löschner, A. Mehner, S. Grund, A. Seifert, A. Pohlers, A. Lange, G. Cox, H.-J. Hähnle and S. Spange, Angew. Chem., Int. Ed., 2012, 51, 3258–3261 CrossRef PubMed.
- S. Spange, P. Kempe, A. Seifert, A. A. Auer, P. Ecorchard, H. Lang, M. Falke, M. Hietschold, A. Pohlers, W. Hoyer, G. Cox, E. Kockrick and S. Kaskel, Angew. Chem., Int. Ed., 2009, 48, 8254–8258 CrossRef CAS PubMed.
- T. Ebert, G. Cox, E. Sheremet, O. Gordan, D. R. T. Zahn, F. Simon and S. Spange, Chem. Commun., 2012, 48, 9867–9869 RSC.
- H.-H. Huang, B. Orler and G. L. Wilkes, Polym. Bull., 1985, 14, 557–564 CrossRef CAS.
- H. H. Huang, B. Orler and G. L. Wilkes, Macromolecules, 1987, 20, 1322–1330 CrossRef CAS.
- G.-D. Kim, D.-A. Lee, J.-W. Moon, J.-D. Kim and J.-A. Park, Appl. Organomet. Chem., 1999, 13, 361–372 CrossRef CAS.
- T. Matsumoto, Advanced Materials '93: Computations, Glassy Materials, Microgravity and Non-Destructive Testing, Newnes, 1995 Search PubMed.
- C.-L. Chiang, C.-C. M. Ma, D.-L. Wu and H.-C. Kuan, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 905–913 CrossRef CAS.
- K. Haraguchi, Y. Usami, K. Yamamura and S. Matsumoto, Polymer, 1998, 39, 6243–6250 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental information, CP-MAS-NMR-spectra, IR-spectra, DSC measurements, TEM images and 1H-NMR-spectra. See DOI: 10.1039/c4cc03640a |
| This journal is © The Royal Society of Chemistry 2014 |