Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A cooperative Pd–Cu system for direct C–H bond arylation†
Mathieu
Lesieur
,
Faïma
Lazreg
and
Catherine S. J.
Cazin
*
EaStCHEM School of Chemistry, University of St Andrews, St Andrews, KY16 9ST, UK. E-mail: cc111@st-andrews.ac.uk
First published on 30th June 2014
Abstract
A novel and efficient method for C–H arylation using well-defined Pd– and Cu–NHC systems has been developed. This process promotes the challenging construction of C–C bonds from arenes or heteroarenes using aryl bromides and chlorides. Mechanistic studies show that [Cu(OH)(NHC)] plays a key role in the C–H activation and is involved in the transmetallation with the Pd–NHC co-catalyst.
Cross-coupling reactions have emerged as a general method for the construction of pharmaceutical scaffolds and natural compounds.1 During the last 30 years, numerous methods for the formation of carbon–carbon bonds using transition metal catalysts2 have been reported: Negishi, Mizoroki–Heck, Stille, Suzuki–Miyaura, Sonogashira and Grignard reagents are the most famous, requiring functionalisation of both coupling partners.2,3 Recently, studies have focused on the unreactive C–H bond and the development of atom-economical and more environmentally friendly catalytic transformations. In particular, systems enabling the formation of biaryl (or aryl-heteroaryl) fragments through non-oxidative direct arylation4 have received significant attention.5 In this area, two challenges exist, in addition to the activation of the C–H bond: homocoupling of the aryl halide must be avoided and the arylation must occur selectively at a single site. Considering the superior reactivity of aryl halides versus that of arenes and the presence of multiple C–H bonds in arenes, selective direct arylation remains a challenge. These issues can be addressed by the presence of directing groups on the arene substrate.6 This strategy leads to increased regioselectivity and reactivity, however, the scope of accessible products is limited as often only the C–H bond ortho to the directing group can be activated. In addition, the need for a directing group often means that additional synthetic steps are required for its incorporation and subsequent removal.7 Synthetic strategies avoiding the need for a directing group have therefore been developed, amongst them Pd systems show significant promise, with challenging substrates such as tosylate derivatives.4,5 In such cases, the C–H activation is often believed to occur through a concerted arene metalation/deprotonation pathway or via a Friedel–Crafts type metallation/rearomatisation sequence leading to a Pd–aryl intermediate, before or after oxidative addition of the aryl halide.5c The resulting intermediate is similar to those found in conventional cross-coupling chemistry. Mori et al. developed a Pd/Cu system for the direct arylation of thiazole,8 while recently, Huang and co-workers reported a Pd–Cu system enabling the arylation of heterocycles using aryl bromides.9 The operative mechanism proposed relies on the presence of a N-donor atom on the substrate, which can coordinate the Cu centre and rearrange into a Cu–heteroaryl intermediate after deprotonation by a base. The heteroaryl fragment is then transferred to the palladium centre, and the aryl–heteroaryl product is reductively eliminated. Despite the elegance of this protocol, the substrate scope is limited to benzo-thiazole, -oxazole and -imidazole derivatives.
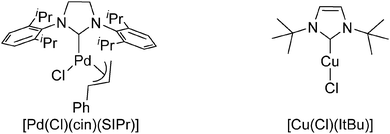
Herein, we report the development of a novel bimetallic catalytic system (Fig. 1) permitting the intermolecular direct arylation of arenes and heteroarenes with aryl and alkenyl bromides and chlorides without the need for a directing group. The working hypothesis is based on the ability of [Cu(OH)(NHC)] to perform C–H activation,10 and the subsequent use of the efficiency of Pd–NHC complexes to allow transmetallation with copper11 after C–Cl or C–Br bond cleavage, leading to the release of the desired compound. In order to validate this hypothesis, pentafluorobenzene and chlorotoluene were selected as coupling partners for the optimisation of reaction conditions. Background reactions were first performed using only Pd–NHC or Cu–NHC, and no conversion was observed in either case (Table 1, entries 1 and 2). Well-defined [Cu(OH)(IPr)] 110a (IPr = N,N′-bis[2,6-(di-iso-propyl)phenyl]imidazol-2-ylidene) complex in combination with [Pd(Cl)(cin)(SIPr)] 212 (SIPr = N,N′-bis[2,6-(di-iso-propyl)phenyl] imidazolidin-2-ylidene; cin = cinnamyl = 3-phenylallyl) represents an initial proof-of-concept in providing the direct arylation product in low conversion (14%, Table 1, entry 3). [Cu(Cl)(IPr)] 313 was next tested as it is likely to be the intermediate species post transmetallation with Pd (see mechanistic discussion below). This chloride derivative leads to the same catalytic activity as its hydroxide congener [Cu(OH)(IPr)] 1 (Table 1, entries 3 and 4), confirming the possible presence of [Cu(Cl)(IPr)] 3 in the catalytic cycle. Several Pd–NHC and [Cu(Cl)(NHC)] complexes were tested,14 and the combination of [Pd(Cl)(cin)(SIPr)] 2 and [Cu(Cl)(ItBu)] 4 (ItBu = N,N′-(di-tert-butyl)imidazol-2-ylidene) showed the best result using CsOH as base and toluene as solvent (Table 1, entry 5).
| Entry | [Pd] (1 mol%) | [Cu] (1 mol%) | Conv.b (%) |
|---|---|---|---|
| a Reaction conditions: pentafluorobenzene (0.75 mmol), 4-chlorotoluene (0.75 mmol), CsOH (0.975 mmol), [Pd(Cl)(cin)(SIPr)] (1 mol%), [Cu(X)(NHC)] (1 mol%) (X = OH or Cl), toluene (3.0 mL), 110 °C, 15 h. b Conversion to the coupling product based on aryl halide determined by GC. c No conversion using 5 mol% Pd. d Isolated yield. | |||
| 1 | [Pd(Cl)(cin)(SIPr)] 2c | — | 0 |
| 2 | — | [Cu(OH)(IPr)] 1 | 0 |
| 3 | [Pd(Cl)(cin)(SIPr)] 2 | [Cu(OH)(IPr)] 1 | 14 |
| 4 | [Pd(Cl)(cin)(SIPr)] 2 | [Cu(Cl)(IPr)] 3 | 13 |
| 5 | [Pd(Cl)(cin)(SIPr)] 2 | [Cu(Cl)(ItBu)] 4 | 93(90)d |
Under the optimised reaction conditions, the scope of this new direct C–H arylation using palladium–NHC and copper–NHC co-catalyst was investigated and the results are presented in Scheme 1. The catalytic system performs equally well with aryl chlorides or bromides, suggesting that the oxidative addition of the aryl halide is not the rate-limiting step in this reaction (7a–c). ortho and meta-substituted aryl halides, with electron donating or withdrawing groups, led to high catalytic activity (7d–h). Different fluoroarene derivatives were studied and the results are included in Scheme 1. Tetrafluoroarenes could be mono-arylated and di-arylated in good yield (7m–o). Trifluoroarenes could also be arylated in good yields (7q–r). 2,3,5,6-Fluoropyridine affords the cross-coupling product in an excellent 98% yield (7p). Highly challenging and sterically congested tetra-ortho-substituted compounds can be synthesised in good isolated yields (7i) using a very bulky NHC palladium complex [Pd(Cl)(cin)(IPr*)] (IPr* = N,N′-bis(2,6-bis(diphenylmethyl)-4-methylphenyl)-imidazo-2-ylidene) developed initially for the preparation of tetra-ortho-substituted biaryls by Suzuki–Miyaura cross-coupling.15 Arylation of α- and β-bromostyrenes was achieved with full conversion and high isolated yields (7j–k). Coupling with an sp3 carbon is also possible as shown by the reaction of pentafluorobenzene with benzyl bromide, leading to a 94% isolated yield of the coupling product (7l). Imidazopyridine could also be efficiently and selectively arylated at the 5-position (7s). C–H arylation of challenging substrates such as 1,4-disubstituted-1,2,3-triazole (7t) is also achieved in good isolated yield (78%) using 1 mol% of Pd and 10 mol% of the copper co-catalyst.
In order to better understand the role played by each metal-complex in the transformation, stoichiometric reactions were carried out (Scheme 2).16 [Cu(C6F5)(IPr)] 8 was obtained quantitatively by the reaction between [Cu(OH)(IPr)] 1 and pentafluorobenzene via C–H activation.17 This possible intermediate species 8 was reacted with [Pd(Cl)(cin)(SIPr)] 2 in the presence of 4-chlorotoluene and CsOH. This led to the concomitant formation of [Cu(Cl)(IPr)] 3 and of the expected coupling product.14
 | ||
| Scheme 2 Stoichiometric reactions.17 | ||
From these observations a proposed catalytic cycle is depicted in Scheme 3. On the copper side of this dual catalytic cycle (left), the first step consists of the in situ formation of the hydroxide [Cu(OH)(NHC)] B from the chloride [Cu(Cl)(NHC)] A in a reaction involving CsOH. The following step consists of the C–H activation of the aryl or heteroaryl via an acid–base reaction, producing [Cu(Ar/Het)(NHC)] C after formation of H2O. At this stage the transmetallation with the Ar′–Pd intermediate D (obtained from oxidative addition of the aryl halide to Pd(0)) occurs, leading concomitantly to the regeneration of [Cu(Cl)(NHC)] A and to the formation of the Ar/Het-Pd-Ar′ intermediate, which can release the coupling product after reductive elimination and regenerate the Pd(0) catalyst.
In conclusion, a dual metal system involving [Cu(Cl)(NHC)] and [Pd(Cl)(cin)(NHC)] has been employed to very effectively perform the direct arylation of C–H bonds without the use of a directing group. The mechanistic studies indicate that the Cu species performs an activation involving acid–base concepts, which then transmetallates to Pd to deliver an aryl or heteroaryl fragment. This methodology is efficient for a broad range of aryl, benzyl and alkenyl bromides and chlorides reacting with aryl and heteroaryl substrates. Ongoing studies focusing on increasing the C–H reactivity of the copper-based system should permit the broadening of the range of coupling partners in this very powerful reaction sequence.
The authors are grateful to the Royal Society (University Research Fellowship to CSJC) for financial support. We also thank the EPSRC National Mass Spectrometry Service Centre in Swansea for High Resolution Mass Spectrometry and Umicore for the generous gift of Pd starting materials.
Notes and references
- J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651 CrossRef CAS PubMed.
- Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2004 Search PubMed.
- (a) A. O. King, N. Okukado and E.-i. Negishi, J. Chem. Soc., Chem. Commun., 1977, 683 RSC; (b) J. E. Milne and S. L. Buchwald, J. Am. Chem. Soc., 2004, 126, 13028 CrossRef CAS PubMed; (c) J. K. Stille, Angew. Chem., Int. Ed. Engl., 1986, 25, 508 CrossRef; (d) P. Espinet and A. M. Echavarren, Angew. Chem., Int. Ed., 2004, 43, 4704 CAS; (e) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (f) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722 CrossRef CAS PubMed; (g) K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467 CrossRef; (h) M. S. Kharasch and C. F. Fuchs, J. Am. Chem. Soc., 1943, 65, 504 CrossRef CAS; (i) M. Yamamura, I. Moritani and S.-I. Murahashi, J. Organomet. Chem., 1975, 91, C39 CrossRef CAS.
- (a) L.-C. Campeau and and K. Fagnou, Chem. Commun., 2006, 1253 RSC; (b) M. Lafrance, C. N. Rowley, T. K. Wo and and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 8754 CrossRef CAS PubMed; (c) M. Lafrance and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 16496 CrossRef CAS PubMed; (d) L. Ackermann and S. Fenner, Chem. Commun., 2011, 47, 430 RSC; (e) L. Ackermann, A. Althammer and S. Fenner, Angew. Chem., Int. Ed., 2009, 48, 201 CrossRef CAS PubMed.
- For reviews, see (a) J. Wencel-Delord, T. Dröge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740 RSC; (b) O. Daugulis, Top. Curr. Chem., 2010, 292, 57 CrossRef CAS; (c) E. M. Beck and M. J. Gaunt, Top. Curr. Chem., 2010, 292, 85 CrossRef CAS; (d) G. P. McGlacken and L. M. Bateman, Chem. Soc. Rev., 2009, 38, 2447 RSC.
- (a) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS PubMed; (b) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed.
- G. Rousseau and B. Breit, Angew. Chem., Int. Ed., 2011, 50, 2450 CrossRef CAS PubMed.
- Example of Pd/Cu systems for direct arylation of heterocycles: A. Mori, A. Sekiguchi, K. Masui, T. Shimada, M. Horie, K. Osakada, M. Kawamoto and T. Ikeda, J. Am. Chem. Soc., 2003, 125, 1700 CrossRef CAS PubMed.
- J. Huang, J. Chan, Y. Chen, C. J. Borths, K. D. Baucom, R. D. Larsen and M. M. Faul, J. Am. Chem. Soc., 2010, 132, 3674 CrossRef CAS PubMed.
- (a) G. C. Fortman, A. M. Z. Slawin and S. P. Nolan, Organometallics, 2010, 29, 3966 CrossRef CAS; (b) F. Lazreg, A. M. Z. Slawin and C. S. J. Cazin, Organometallics, 2012, 31, 7969 CrossRef CAS; (c) S. Gaillard, C. S. J. Cazin and S. P. Nolan, Acc. Chem. Res., 2012, 45, 778 CrossRef CAS PubMed.
- M. R. L. Furst and C. S. J. Cazin, Chem. Commun., 2010, 46, 6924 RSC.
- N. Marion, O. Navarro, J. Mei, E. D. Stevens, N. M. Scott and S. P. Nolan, J. Am. Chem. Soc., 2006, 128, 4101 CrossRef CAS PubMed.
- C. A. Citadelle, E. Le Nouy, F. Bisaro, A. M. Z. Slawin and C. S. J. Cazin, Dalton Trans., 2010, 39, 4489 RSC.
- See ESI† for more details.
- A. Chartoire, M. Lesieur, L. Falivene, A. M. Z. Slawin, L. Cavallo, C. S. J. Cazin and S. P. Nolan, Chem. – Eur. J., 2012, 18, 4517 CrossRef CAS PubMed.
- N. M. Scott and S. P. Nolan, Eur. J. Inorg. Chem., 2005, 1815 CrossRef CAS.
- The reaction involving 1 was also successfully carried out in toluene, but requires a longer reaction time.
Footnote |
| † Electronic supplementary information (ESI) available: Catalyst optimisation, mechanistic studies and NMR spectra of all products. See DOI: 10.1039/c4cc03201b |
| This journal is © The Royal Society of Chemistry 2014 |