 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sulfated quaternary amine lipids: a new class of inverse charge zwitterlipids†
Vincent J.
Venditto
a,
Aaron
Dolor
a,
Aditya
Kohli
b,
Stefan
Salentinig
c,
Ben J.
Boyd
c and
Francis C.
Szoka
Jr.
*ab
aDepartment of Bioengineering and Therapeutic Sciences, School of Pharmacy, University of California San Francisco, San Francisco, CA 94143, USA
bThe UC Berkeley-UCSF Graduate Program in Bioengineering, University of California Berkeley, Berkeley, CA 94720, USA. E-mail: szoka@cgl.ucsf.edu
cDrug Delivery, Disposition and Dynamics, Monash Institute of Pharmaceutical Sciences, Monash University, Victoria 3052, Australia
First published on 23rd June 2014
Abstract
We describe a new class of charge inverted zwitterionic sulfated lipids (AS) with a cationic quaternary amine anchored at the membrane interface and an anionic sulfate moiety extended into the aqueous phase. These lipids have exceptionally high transition temperatures and assemble into lipid aggregates when dispersed in aqueous solutions.
Naturally occurring zwitterionic lipids (e.g. phosphatidylcholine (PC) and phosphatidyl ethanolamine (PE)) contain headgroups with an anionic moiety at the bilayer interface and a cationic charge extended into the aqueous phase. Recently, we reported inverse charge zwitterlipids containing carboxylates (AQ),1,2 phosphates (CP)3,4 and sulfonates (SB).5 The inverse charges of these lipids have been shown to alter the physical properties of the lipids as compared to normal phospholipids. CP and CPe lipids formed small, stable vesicles with sonication, while AQ and SB lipids only formed liposomes when co-formulated with cholesterol or hydrated with chaotropic salts, respectively. Liposomes prepared from each of the previously reported inverse zwitterlipids possessed a weaker association for divalent cations (e.g. Ca2+), suggesting that these inverse charge lipids may have potential for use in drug delivery.4
To further explore the structure–function properties of inverse charge zwitterlipids we incorporated sulfate as the anionic species. Sulfation is a common post-synthetic modification that occurs in carbohydrates,6 proteins7 and lipids.8 For instance, lipid sulfation of galactosyl ceramide is required for proper development of myelin while seminolipid sulfation is necessary for spermatogenesis.8 In both cases the lipids contain carbohydrate headgroups, which are sulfated to produce anionic headgroups. Other synthetic sulfated lipids have been prepared as detergents, as exemplified by sodium dodecyl sulfate (SDS). Our recent interest in inverse charge zwitterionic lipids,1–3,5 however, suggested that lipid sulfation would be a viable alternative to the inverse charge PC lipids (CP).
From a design perspective, sulfate introduces a single negative charge with a low pKa as compared to the dianionic charge of phosphate. The CP inverse phospholipid contains an amine and a phosphate, which results in a net negatively charged lipid at pH 7.0. Here, we prepare a series of inverse ammonium sulfate lipids (AS) through a straightforward synthesis shown in Scheme 1. The sulfate headgroup was achieved through sulfation of bromopropanol with sulfurtrioxide–pyridine to afford bromopropylsulfate. Subsequently, 3-(dimethylamino)-1,2-propanediol was acylated with a variety of lipid tails and the resulting products (2a–f) were alkylated with bromopropylsulfate (1) at the amine to afford the final products (3a–f) containing a quaternary amine and pendant sulfate. Upon quaternization of the amine, most of the lipids (3b–3f) precipitated from DMF at 60 °C as pure products. Additional recrystallization was performed to remove minor impurities. DOAS (3a) failed to precipitate in DMF and was purified by silica gel chromatography.
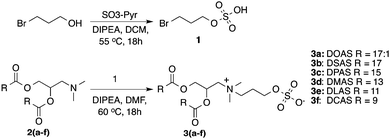 | ||
| Scheme 1 Synthetic scheme to synthesize a novel series of sulfated quaternary amine charge inverted zwitterionic lipids (AS). | ||
Each of the pure lipids had exceptionally high transition temperatures as measured by differential scanning calorimetry (Fig. 1). The Tm determined for each lipid is 38, 89, 83, 76, 67, and 56 °C for DOAS (3a), DSAS (3b), DPAS (3c), DMAS (3d), DLAS (3e) and DCAS (3f), respectively. The elevated Tm observed with the AS series of lipids are higher than those observed for the other inverse lipids. CP and CPe lipids maintain the Tm of phosphatidylcholine lipids, AQ and SB lipids have elevated Tm, resembling phosphatidylethanolamine lipids. To the best of our knowledge, this is the first report of a cis-unsaturated lipid with a transition temperature above 25 °C. Furthermore, phase transition temperatures of all six lipids are 30–60 °C higher than the PC lipids of corresponding chain length (Fig. 1). The high transition temperature observed for these lipids as compared to traditional phosphatidylcholine lipids and even phosphatidylethanolamine lipids suggest that the sulfate–amine interactions in the headgroup have a significant effect on the gel to liquid phase transition.
Based on the potential intra/intermolecular headgroup interactions, we investigated the effect of chaotropic salts on transition temperature using the Hofmeister series (ClO4− > I− > Cl−). In the inverse sulfobetaine (SB) lipids, chaotropic salts reduce the transition temperatures by interacting with the polar headgroups and perhaps interfering with van der Waals interactions in the lipid tails. AS lipids behave similarly (Fig. 2A–C): high concentration of sodium perchlorate decreased the transition temperature of each lipid by about 30 °C. Yet the transition temperatures still remain higher than the analogous phospholipids. Sodium iodide also affected the Tm of each lipid causing two distinct lower temperature transitions to appear; the lowest at the same temperature as observed with perchlorate. Furthermore, kosmotropic salts (NH4Cl, Na2SO4, and (NH4)2SO4) do not induce the same elevated temperatures seen with PC lipids (Fig. S27, ESI†), which we attribute to a covalent “salting out” effect with the kosmotropic ions of the head group.
Increasing cholesterol content was then found to decrease the enthalpy of the DSAS lipid transition (Fig. 2D) as seen with traditional PC lipids. The presence of the main transition at 50 mol% cholesterol is due to the inability to prepare homogenous lipid formulations, which leads to phase separated domains. Preparation of homogenous DMAS formulations with cholesterol is equally challenging and the resulting formulations exhibit similar effects with cholesterol.
The heterogeneous nature of the lipid formulations made with DMAS also prevented appropriate small angle X-ray scattering analysis to quantify the bilayer dimensions. DOAS, on the other hand, was more easily analysed and found to have a bilayer spacing of about 46 Å, which is in close agreement with DOPC bilayer spacing of 49.1 Å for DOPC.9
We then attempted to prepare nanoparticles using these lipids to evaluate their behaviour and stability. We focused our efforts on two lipids, DOAS (3a) and DMAS (3d), due to their lower transition temperatures and the ability of analogous PC lipids to form stable vesicles. Lipids were rehydrated in buffer in the presence or absence of carboxyfluorescein. Both lipids were well hydrated above their respective transition temperatures, and precipitated to the bottom of the tube when cooled below the transition temperature. Neither formulation encapsulated CF. Transmission electron microscopy of the DOAS formulation revealed small (<100 nm) worm-like structures and spirals (Fig. 3). In the TEM of the DMAS formulation there were particles of various sizes that were stacked or interacting with other particles. The zeta potential of all lipid dispersions was neutral at pH 7.4.
 | ||
| Fig. 3 TEM images of formulations prepared with DOAS, DMAS, DMAS with 10% tocopherol (DMAS:TC) and DMAS with 10% tocopherol-PEG (DMAS:TC-PEG). | ||
Various attempts to prepare vesicles using additives (e.g. salts or sucrose), and coformulations with helper lipids (e.g. cholesterol and other long chain lipids) also failed to enable the formation of lipid vesicles. When DSPE-PEG was added to the preparation, the formulation could encapsulate CF, as evidenced by the elution of the dye in the excluded volume of a size exclusion Sephadex G-25 column, but rapidly leaked from the eluted mixture at room temperature.
We then also included lipids that might induce positive curvature in the bilayer including tocopherol (TC) and PEGylated tocopherol (TC-PEG). When formulated with CF, both formulations leaked CF rapidly at 25 °C. TEM images of each formulation showed dramatically different structures with DMAS:TC generating large (1 μm) amorphous looking structures with poorly defined edges, while DMAS:TC-PEG generated sheet-like structures (Fig. 3). Furthermore, all lipid dispersions maintained neutral zeta potentials.
We have synthesized a series of novel inverse-charge zwitterionic sulfolipids with exceptionally high transition temperatures. The high phase transition temperatures are partly due to the sulfate–amine interactions within the headgroup, which are affected by chaotropic salts. We believe that the inability to form stable vesicles using sulfolipids stems from their charge interactions at the aqueous-bilayer interface and their high transition temperatures. In aqueous solutions, the sulfated quaternary amine lipids appear to form bilayer sheets but do not form stable vesicular structures. The lipids join a family of inverse lipids with properties that differ significantly from the phosphatidylcholines of comparable chain length.10
This work was supported by NIH-EB-003008, an NIH Ruth L Kirshchstein National Research Service Award 1F32AI095062 and the National Science Foundation Graduate Research Fellowship Program. The authors would also like to thank Reena Zalpuri and Olivier Julien for assistance in obtaining transmission electron microscopy images. We would also like to thank reviewer #1 for suggesting the experiments with kosmotropic ions leading to a covalent “salting out” effect.
Notes and references
- A. G. Kohli, C. L. Walsh and F. C. Szoka, Chem. Phys. Lipids, 2012, 165, 252–259 CrossRef CAS PubMed.
- C. L. Walsh, J. Nguyen and F. C. Szoka, Chem. Commun., 2012, 48, 5575–5577 RSC.
- E. K. Perttu, A. G. Kohli and F. C. Szoka Jr, J. Am. Chem. Soc., 2012, 134, 4485–4488 CrossRef CAS PubMed.
- J. Nguyen, C. L. Walsh, J. P. M. Motion, E. K. Perttu and F. Szoka, Pharm. Res., 2012, 29, 2236–2248 CrossRef CAS PubMed.
- E. K. Perttu and F. C. Szoka, Chem. Commun., 2011, 47, 12613–12615 RSC.
- J. Kreuger and L. Kjellén, J. Histochem. Cytochem., 2012, 60, 898–907 CrossRef PubMed.
- J. W. Kehoe and C. R. Bertozzi, Chem. Biol., 2000, 7, R57–R61 CrossRef CAS.
- K. Honke, Y. Zhang, X. Cheng, N. Kotani and N. Taniguchi, Glycoconjugate J., 2004, 21, 59–62 CrossRef CAS.
- R. E. Jacobs and S. H. White, Biochemistry, 1989, 28, 3421–3437 CrossRef CAS.
- A. G. Kohli, P. H. Kierstead, V. J. Venditto, C. L. Walsh and F. C. Szoka, J. Controlled Release, 2014 DOI:10.1016/j.jconrel.2014.04.047 , Epub ahead of print.
Footnote |
| † Electronic supplementary information (ESI) available: Including synthesis, characterization and a list of lipid formulations prepared and analyzed. See DOI: 10.1039/c4cc02866j |
| This journal is © The Royal Society of Chemistry 2014 |


