Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dynamic nuclear polarisation NMR of nanosized zirconium phosphate polymer fillers†
Fabio
Ziarelli
a,
Mario
Casciola
b,
Monica
Pica
b,
Anna
Donnadio
b,
Fabien
Aussenac
c,
Claire
Sauvée
d,
Donatella
Capitani
*e and
Stéphane
Viel
 *d
*d
aAix Marseille Université, Centrale Marseille, CNRS, Fédération des Sciences Chimiques FR 1739, F-13397 Marseille, France
bUniversità degli studi di Perugia, Dipartimento di Chimica – CEMIN, I-06123 Perugia, Italy
cBruker Biospin SAS, F-67160 Wissembourg, France
dAix Marseille Université, CNRS, ICR UMR 7273, F-13397 Marseille, France. E-mail: s.viel@univ-amu.fr
eLaboratorio NMR “Annalaura Segre”, Istituto di Metodologie Chimiche, CNR, 00015 Monterotondo (Roma), Italy. E-mail: donatella.capitani@cnr.it
First published on 15th July 2014
Abstract
Surface functionalisation with organic modifiers of multi-layered zirconium phosphate (ZrP) nanoparticles used as polymer fillers can be directly probed by dynamic nuclear polarisation NMR, which provides unambiguous evidence of the presence of P–O–C chemical bonds at the surface of the ZrP layers, thereby confirming successful functionalisation.
α-Type monohydrogen zirconium phosphate (ZrP) particles are made by the packing of planes of zirconium atoms sandwiched between monohydrogen phosphate groups (hereafter referred to as ZrP layers), which are held together by weak van der Waals interactions. ZrP particles are promising inorganic polymer fillers that can be used either with charged or neutral polymers depending on the chemical properties of the surface of their constituting layers. Precisely, the presence of POH groups on the surface of the layers favours the use of ZrP particles with polymers possessing polar groups (e.g. starch),1 whereas the surface of the layers must be organically functionalised when using ZrP particles with neutral polymers, so as to promote stronger interactions with the polymer matrix.2,3 Accurate chemical characterization of the surface of the ZrP layers is hence tantamount to controlling the ultimate properties of the ZrP particles, and this is usually achieved indirectly through a combination of techniques, such as infrared spectroscopy, thermogravimetric analysis, and solid-state NMR (SSNMR). In principle, SSNMR alone could provide the required information with almost atomic resolution, but its intrinsically low sensitivity precludes direct analysis of diluted signals, such as those arising from the surface of materials. Therefore, direct (and unambiguous) assessment of surface functionalisation of nanosized ZrP polymer fillers remains highly challenging. Dynamic nuclear polarisation surface enhanced NMR spectroscopy (DNP SENS)4 could potentially circumvent this difficulty. DNP enhances the sensitivity of SSNMR experiments through the microwave driven transfer of polarisation of unpaired electrons to nuclei.5–9 The particularity of the DNP SENS approach lies in its ability to probe the surface species. By incipiently wetting the solid with a radical containing solution, the polarisation gets transferred upon microwave irradiation from the unpaired electrons of the radicals (the so-called DNP polarising agents) to the nearby protons of the solvent molecules. This enhanced 1H magnetization then spreads out through the medium by spin diffusion and eventually reaches the vicinity of the material surface, where it is transferred to a heteronucleus of interest by cross polarisation techniques. While DNP SENS has already been used in a range of applications,4 including for the investigation of nanoparticles,10–12 it has never been applied, to the best of our knowledge, for probing the presence of chemical covalent bonds on the surface of nanoparticles.
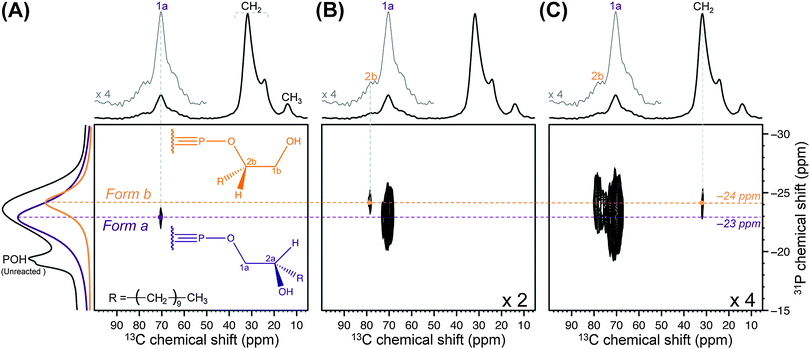
Herein, DNP SENS was used to characterize nanosized ZrP polymer fillers obtained by reacting ZrP gels with 1,2-epoxydodecane,13 which holds promise as a mechanical strengthener for polymer electrolyte membranes.14 Specifically, the sensitivity enhancement provided by DNP allowed two-dimensional (2D) 31P–13C dipolar correlation experiments15 at natural 13C abundance (∼1%) to be carried out on diluted ZrP samples (the amount of alkyl chains grafted onto ZrP nanoparticles was ∼3.5 μmol mg−1), experiments that are unfeasible using conventional SSNMR instrumentation due to lack of sensitivity. Dipolar correlation experiments directly evidence dipolar contacts – hence, spatial proximities – between the nuclei under investigation. Combining these data with the observation of specific chemical shifts in the DNP-enhanced 13C and 31P CPMAS spectra is shown here to provide direct and unambiguous evidence for the presence of P–O–C bonds at the surface of the ZrP layers. In particular, the ZrP sample functionalised with 1,2-epoxydodecane was prepared by incipient wetness impregnation16 (see ESI†) using a 14 mM aqueous solution of a recently described dinitroxide (i.e. PyPOL).17 Dinitroxides are currently the most efficient DNP polarizing agents for high-resolution DNP SSNMR experiments.17,18 The first indication of the sensitivity gain afforded by DNP is illustrated in Fig. 1, where the DNP-enhanced 31P and 13C CPMAS spectra of the functionalised ZrP sample are reported. Comparing the signal intensities of the spectra obtained with the microwave field on (ION) and off (IOFF), led to a sensitivity increase (εX,CP = ION/IOFF, where X is 31P or 13C) of 6 and 8, respectively.‡ Precisely, upon comparing the signal-to-noise ratio (S/N) per unit of time of the DNP SSNMR experiment with the S/N per unit of time of the SSNMR experiment without DNP (at room temperature), the so-called absolute sensitivity ratio (ASR),19,20 an ASR value of 10 was obtained, which translated into a time saving factor of 100. This substantial sensitivity enhancement§ allowed us to perform the 31P–13C SSNMR dipolar correlation experiment shown in Fig. 2 within a realistic experimental time frame (∼2 days), while the same experiment would have prohibitively required more than 200 days on comparable SSNMR instrumentation without DNP and at room temperature.
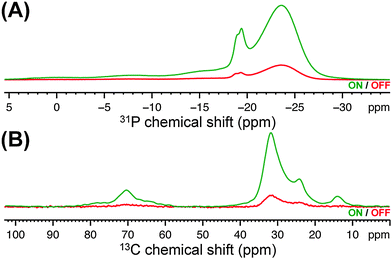 | ||
| Fig. 1 DNP-enhanced (A) 31P and (B) 13C CPMAS spectra of the functionalised ZrP sample with the microwave field on and off. | ||
The reaction between 1,2-epoxydodecane and the POH groups of crystalline ZrP may lead to the formation of 2 types of functional groups (Forms a and b in the inset of Fig. 2A).13 For ZrP nanoparticles, spectral deconvolution of the DNP-enhanced 31P CPMAS spectrum (Fig. 1A) confirmed the presence of two main forms in an approximate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
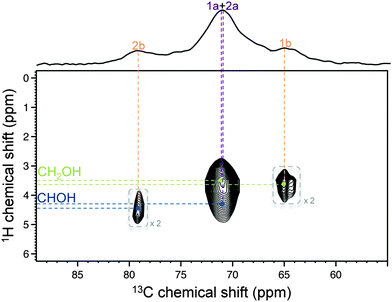
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (at −23 and −24 ppm, respectively), and revealed the presence of a few minor 31P resonances due to the unreacted species (see ESI†). Based on the assignment of organically modified crystalline ZrP,13 these 31P resonances could be attributed to Forms a and b, respectively. Most importantly, the correlation between δ(31P) −23 ppm and δ(13C) 71 ppm (Fig. 2A), and the correlation between δ(31P) −24 ppm and δ(13C) 79 ppm (Fig. 2B), were clear indications of the presence of covalent P–O–C bonds, thereby unambiguously proving the successful grafting of the alkyl chains onto the surface of the ZrP layers of the nanoparticles. Moreover, because the dipolar interaction falls off very quickly with the internuclear distance, correlations involving shorter internuclear distances are typically predominant in dipolar correlation experiments. As a result, these correlations must be related to the 13C nuclei that are closest to the 31P nuclei, which allowed us to assign the 13C chemical shifts at 71 and 79 ppm to carbons 1a and 2b, respectively. This partial assignment was confirmed and completed by the 1H–13C HETCOR experiment reported in Fig. 3 (recorded without DNP because sensitivity was not an issue in this case). The values of 1H chemical shifts observed in this spectrum allowed us to confirm the above assignment and to assign the last two 13C signals at 71 ppm (due to a CH group, hence carbon 2a) and at 65 ppm (due to a CH2 group, hence carbon 1b). Overall, the resulting assignment was found to be in full agreement with the spectral deconvolution of the 50–100 ppm region of the DNP-enhanced 13C CPMAS spectrum shown in Fig. 1B (see ESI†). Finally, in Fig. 2C, another correlation involving Form b at δ(13C) 31 ppm could be detected, which was due to the 13C signal of the first CH2 group of the alkyl chain. This correlation was not observed for Form a. This is consistent with the fact that the distance between this CH2 group and the 31P nuclei of the P–O–C bonds (at the surface of the ZrP layers) is shorter in Form b than in Form a. More importantly, no other 31P–13C dipolar correlation involving the 13C nuclei of the alkyl chains could be observed. This suggested that the alkyl chains were not lying on the surface of the ZrP layers but exhibited an extended conformation that spreads away from the surface of the layers. This point is critical because it indicates that the grafting has potentially rendered the (originally polar) surface of the ZrP layers more hydrophobic, a necessary prerequisite when using ZrP nanoparticles as fillers for aliphatic polymers.
1 ratio (at −23 and −24 ppm, respectively), and revealed the presence of a few minor 31P resonances due to the unreacted species (see ESI†). Based on the assignment of organically modified crystalline ZrP,13 these 31P resonances could be attributed to Forms a and b, respectively. Most importantly, the correlation between δ(31P) −23 ppm and δ(13C) 71 ppm (Fig. 2A), and the correlation between δ(31P) −24 ppm and δ(13C) 79 ppm (Fig. 2B), were clear indications of the presence of covalent P–O–C bonds, thereby unambiguously proving the successful grafting of the alkyl chains onto the surface of the ZrP layers of the nanoparticles. Moreover, because the dipolar interaction falls off very quickly with the internuclear distance, correlations involving shorter internuclear distances are typically predominant in dipolar correlation experiments. As a result, these correlations must be related to the 13C nuclei that are closest to the 31P nuclei, which allowed us to assign the 13C chemical shifts at 71 and 79 ppm to carbons 1a and 2b, respectively. This partial assignment was confirmed and completed by the 1H–13C HETCOR experiment reported in Fig. 3 (recorded without DNP because sensitivity was not an issue in this case). The values of 1H chemical shifts observed in this spectrum allowed us to confirm the above assignment and to assign the last two 13C signals at 71 ppm (due to a CH group, hence carbon 2a) and at 65 ppm (due to a CH2 group, hence carbon 1b). Overall, the resulting assignment was found to be in full agreement with the spectral deconvolution of the 50–100 ppm region of the DNP-enhanced 13C CPMAS spectrum shown in Fig. 1B (see ESI†). Finally, in Fig. 2C, another correlation involving Form b at δ(13C) 31 ppm could be detected, which was due to the 13C signal of the first CH2 group of the alkyl chain. This correlation was not observed for Form a. This is consistent with the fact that the distance between this CH2 group and the 31P nuclei of the P–O–C bonds (at the surface of the ZrP layers) is shorter in Form b than in Form a. More importantly, no other 31P–13C dipolar correlation involving the 13C nuclei of the alkyl chains could be observed. This suggested that the alkyl chains were not lying on the surface of the ZrP layers but exhibited an extended conformation that spreads away from the surface of the layers. This point is critical because it indicates that the grafting has potentially rendered the (originally polar) surface of the ZrP layers more hydrophobic, a necessary prerequisite when using ZrP nanoparticles as fillers for aliphatic polymers.
In summary, DNP allows the surface of polymer fillers to be characterized, and hence appears to be highly relevant for studying the filler/polymer interface in polymeric nanocomposites. This technique is clearly not limited to ZrP nanoparticles and should be useful for characterizing the surface functionalisation of other types of phosphate materials.
This work has been carried out thanks to the support of the A*MIDEX project (no. ANR-11-IDEX-0001-02) funded by the “Investissements d'Avenir” French Government program, managed by the French National Research Agency (ANR).
Notes and references
- M. Pica, A. Donnadio, V. Bianchi, S. Fop and M. Casciola, Carbohydr. Polym., 2013, 97, 210–216 CrossRef CAS PubMed.
- L. Y. Sun, W. J. Boo, D. H. Sun, A. Clearfield and H. J. Sue, Chem. Mater., 2007, 19, 1749–1754 CrossRef CAS.
- B. M. Mosby, A. Diaz, V. Bakhmutov and A. Clearfield, ACS Appl. Mater. Interfaces, 2014, 6, 585–592 CAS.
- A. J. Rossini, A. Zagdoun, M. Lelli, A. Lesage, C. Copéret and L. Emsley, Acc. Chem. Res., 2013, 46, 1942–1951 CrossRef CAS PubMed.
- A. W. Overhauser, Phys. Rev., 1953, 92, 411–416 CrossRef CAS.
- T. Carver and C. P. Slichter, Phys. Rev., 1953, 92, 212–213 CrossRef CAS.
- L. R. Becerra, G. J. Gerfen, R. J. Temkin, D. J. Singel and R. G. Griffin, Phys. Rev. Lett., 1993, 71, 3561–3564 CrossRef CAS.
- D. A. Hall, D. C. Maus, G. J. Gerfen, S. J. Inati, L. R. Becerra, F. W. Dahlquist and R. G. Griffin, Science, 1997, 276, 930–932 CrossRef CAS.
- Q. Z. Ni, E. Daviso, T. V. Can, E. Markhasin, S. K. Jawla, T. M. Swager, R. J. Temkin, J. Herzfeld and R. G. Griffin, Acc. Chem. Res., 2013, 46, 1933–1941 CrossRef CAS PubMed.
- L. Protesescu, A. J. Rossini, D. Kriegner, M. Valla, A. de Kergommeaux, M. Walter, K. V. Kravchyk, M. Nachtegaal, J. Stangl, B. Malaman, P. Reiss, A. Lesage, L. Emsley, C. Copéret and M. V. Kovalenko, ACS Nano, 2014, 8, 2639–2648 CrossRef CAS PubMed.
- O. Lafon, A. S. L. Thankamony, M. Rosay, F. Aussenac, X. Y. Lu, J. Trebosc, V. Bout-Roumazeilles, H. Vezin and J. P. Amoureux, Chem. Commun., 2013, 49, 2864–2866 RSC.
- U. Akbey, B. Altin, A. Linden, S. Ozcelik, M. Gradzielski and H. Oschkinat, Phys. Chem. Chem. Phys., 2013, 15, 20706–20716 RSC.
- M. Casciola, D. Capitani, A. Donnadio, G. Munari and M. Pica, Inorg. Chem., 2010, 49, 3329–3336 CrossRef CAS PubMed.
- A. Donnadio, M. Pica, D. Capitani and M. Casciola, J. Membr. Sci., 2014, 462, 42–49 CrossRef CAS PubMed.
- J. Schaefer, R. A. McKay and E. O. Stejskal, J. Magn. Reson., 1979, 34, 443–447 CAS.
- A. Lesage, M. Lelli, D. Gajan, M. A. Caporini, V. Vitzthum, P. Mieville, J. Alauzun, A. Roussey, C. Thieuleux, A. Mehdi, G. Bodenhausen, C. Coperet and L. Emsley, J. Am. Chem. Soc., 2010, 132, 15459–15461 CrossRef CAS PubMed.
- C. Sauvée, M. Rosay, G. Casano, F. Aussenac, R. T. Weber, O. Ouari and P. Tordo, Angew. Chem., Int. Ed., 2013, 52, 10858–10861 CrossRef PubMed.
- A. Zagdoun, G. Casano, O. Ouari, M. Schwarzwälder, A. J. Rossini, F. Aussenac, M. Yulikov, G. Jeschke, C. Copéret, A. Lesage, P. Tordo and L. Emsley, J. Am. Chem. Soc., 2013, 135, 12790–12797 CrossRef CAS PubMed.
- H. Takahashi, D. Lee, L. Dubois, M. Bardet, S. Hediger and G. De Paëpe, Angew. Chem., Int. Ed., 2012, 51, 11769 Search PubMed.
- H. Takahashi, B. Viverge, D. Lee, P. Rannou and G. De Paëpe, Angew. Chem., Int. Ed., 2013, 52, 6979–6982 CrossRef CAS PubMed.
- S. C. Christiansen, N. Hedin, J. D. Epping, M. T. Janicke, Y. del Amo, M. Demarest, M. Brzezinski and B. F. Chmelka, Solid State Nucl. Magn. Reson., 2006, 29, 170–182 CrossRef CAS PubMed.
- T. Kobayashi, O. Lafon, A. S. L. Thankamony, I. I. Slowing, K. Kandel, D. Carnevale, V. Vitzthum, H. Vezin, J. P. Amoureux, G. Bodenhausen and M. Pruski, Phys. Chem. Chem. Phys., 2013, 15, 5553–5562 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental protocols. See DOI: 10.1039/c4cc02723j |
| ‡ These values are substantially lower than those expected with this DNP polarising agent,17 which could be related to the possibly inhomogeneous glass formed upon cooling the sample at ∼100 K (presence of water crystals in this case) and/or to a nonuniform dispersion of the radicals in the sample, which are both deleterious to an efficient DNP transfer. In fact, 1H relaxation data reported in the SI suggest that biradicals were not homogeneously dispersed in the sample. |
| § This sensitivity enhancement might even be further increased using alternative double cross-polarisation21 and/or detection strategies.22 |
| This journal is © The Royal Society of Chemistry 2014 |