Open Access Article
Open Access ArticleBiomass derived furfural-based facile synthesis of protected (2S)-phenyl-3-piperidone, a common intermediate for many drugs†
P.-F.
Koh
a,
P.
Wang
a,
J.-M.
Huang
ab and
T.-P.
Loh
*ac
aDivision of Chemistry & Biological Chemistry, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore
bSchool of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong 510640, China
cHefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026, P. R. China. E-mail: teckpeng@ntu.edu.sg
First published on 1st May 2014
Abstract
An efficient synthetic route towards tosyl-protected (2S)-phenyl-3-piperidone, a common intermediate for many drugs, has been developed in 5 steps in 54% yield from biomass derived furfural. The synthetic utility of the piperidone core structure was demonstrated with the synthesis of a NK1 receptor antagonist.
The sustained increase in the consumption of finite fossil fuel resources has painted a bleak global energy outlook for the 21st century. This has also attracted considerable research attention to various renewable resources such as biomass, which has the potential to serve as a renewable source of energy and organic carbon.1 Furfural 1 is a platform chemical which can be derived from biomass2a,b and annually about 300
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of agricultural raw materials are dehydrated to form furfural. It is notable that a recent report suggests a possible significant reduction in furfural production costs2c which highlights the potential for lower costs when utilizing furfural as a carbon source. The inclusion of furfural as one of the top “biobased product opportunities”3a emphasizes its usefulness in various domains such as fuels,3b solvents,3c natural product synthesis3d–i and more recently as chiral inducers.3j Annual world production of rice exceeds 500 million tonnes4a and its associated agricultural waste, rice straw, is produced in large quantities in Asian countries such as China (110 Mt per year), India (97 Mt per year), Thailand (22 Mt per year) and the Philippines (11 Mt per year).4b,c Currently rice straw is largely left uncollected in the field or is disposed of through open-field burning which causes air pollution and health hazards.4
000 tonnes of agricultural raw materials are dehydrated to form furfural. It is notable that a recent report suggests a possible significant reduction in furfural production costs2c which highlights the potential for lower costs when utilizing furfural as a carbon source. The inclusion of furfural as one of the top “biobased product opportunities”3a emphasizes its usefulness in various domains such as fuels,3b solvents,3c natural product synthesis3d–i and more recently as chiral inducers.3j Annual world production of rice exceeds 500 million tonnes4a and its associated agricultural waste, rice straw, is produced in large quantities in Asian countries such as China (110 Mt per year), India (97 Mt per year), Thailand (22 Mt per year) and the Philippines (11 Mt per year).4b,c Currently rice straw is largely left uncollected in the field or is disposed of through open-field burning which causes air pollution and health hazards.4
We envisioned that the xylan content present in rice straw could be used as a feedstock to produce furfural which can then be efficiently transformed into a tosyl-protected (2S)-phenyl-3-piperidone core structure 2 which allows facile access to numerous neurokinin-1 (NK1) receptor antagonists (Fig. 1). These potent NK1 receptor antagonists showed promising biological activities which may offer novel cures to disorders such as depression, anxiety and emesis.5 Various protected 2-phenyl-3-piperidones have been synthesized by Merck and other research groups with low overall yields (<40%) over a minimum of 6 steps.6,7 The advantages of this strategy include the use of a cheap and renewable biomass-derived starting material, being a short synthetic route with only a single silica gel column chromatography step, and obtaining product in a higher yield than existing methods with almost no loss of optical purity.
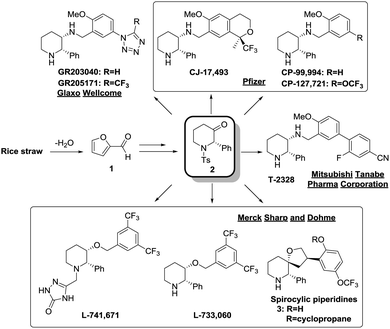 | ||
| Fig. 1 Construction of the piperidone core structure from furfural to access various potent NK1 receptor antagonists. | ||
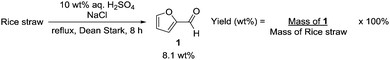
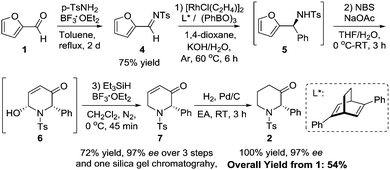
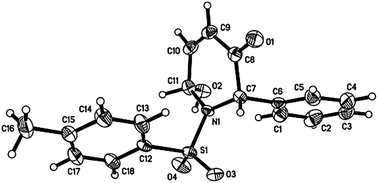
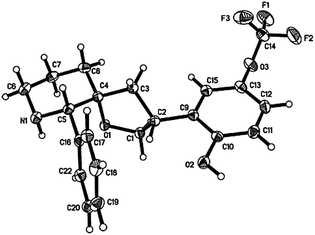
Modification of a previously reported synthesis of furfural 1 from corn cobs8 to rice straw with hourly removal of DCM from the Dean Stark trap gave a yield of 8.1 wt% without optimization (Scheme 1). The crude product obtained was found to be sufficiently pure without the need for further purification and could be transformed into imine 4 in the presence of 4-methylbenzenesulfonamide and a Lewis acid catalyst with a recrystallization yield of 75% (Scheme 2). Imine 4 was then subjected to a rhodium-catalyzed asymmetric arylation methodology developed by Hayashi's group9 to afford furylamine 5 with 97% yield and 99% enantiomeric excess (ee) after column chromatography. In view of the efficiency of this step, subsequent reactions were not subjected to chromatographic purification and crude 5 was able to undergo the aza-Achmatowicz rearrangement10 with N-bromosuccinimide (NBS) as the oxidant to yield hemiaminal 6 as a cis diastereomer, as determined by NMR analysis and single-crystal X-ray crystallography11 (Fig. 2), probably as a result of an anomeric effect. The phenyl substituent at the chiral centre in rac-6 adopts a pseudoaxial orientation due to A1,3-strain12 with the tosyl protecting group. The aza-Achmatowicz rearrangement is a variation of the Achmatowicz rearrangement where the former involves an amine functional group as a nucleophile and the latter an alcohol. Crude 6 was able to be immediately reduced13 without further purification to give 7 in 72% yield and 97% ee over 3 steps from 4. 7 was hydrogenated using Pd/C in a quantitative conversion to yield tosyl-protected (2S)-phenyl-3-piperidone 2. Thus, key intermediate 2 was efficiently synthesized in an overall yield of 54% over 5 steps from rice straw-derived furfural 1.
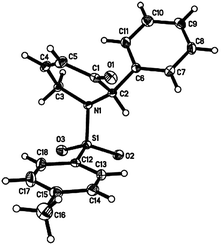
The optical rotation obtained for 7 is [α]21D = +123 (c = 1.32, CH2Cl2) for 97% ee while that reported in the literature7c is [α]20D = −145 (c = 0.3, CH2Cl2), and the optical rotation obtained for 2 is [α]23D = −10.0 (c = 1.01, CH2Cl2) for 97% ee while that reported in the literature7c is [α]20D = +5 (c = 0.2, CH2Cl2). The absolute structure of 7 was determined using single-crystal X-ray crystallography11 (Fig. 3), and further HPLC analysis of the particular single-crystal used in the X-ray crystallography as well as the batch of crystals submitted for analysis showed retention times and an elution order that were in agreement with those of experimentally obtained values (see ESI† for more details). This rules out the possibility that the structure obtained from the single-crystal X-ray crystallography is the minor enantiomer and hence it can be concluded that the experimentally obtained 7 is indeed the desired (S)-2-phenyl-1-tosyl-1,6-dihydropyridin-3(2H)-one. This conclusion can be further extended to assign the absolute configuration of 2 as (S)-2-phenyl-1-tosylpiperidin-3-one. The loss of ee was subsequently determined to be due to the inherent acidity of the silica gel chromatography; pre-treatment of silica gel with 1% triethylamine also resulted in a loss of ee while recrystallization attempts proved to be futile. The acid and base sensitivity of 2 and 7 may be attributed to the lability of the α-hydrogen at the chiral centre.
NK1 receptor antagonist 3 was synthesized to illustrate the synthetic utility of piperidone 2 (Scheme 3). Rac-2 was able to undergo a Grignard reaction and subsequent TMS deprotection step to form rac-8, which was immediately subjected to Searles–Crabbé homologation14 conditions without further purification to transform the alkyne moiety to an allene rac-9 in 69% yield over 3 steps. The relative stereochemistry in rac-8 was established using single-crystal X-ray crystallography11 where the alkyne is trans to the phenyl substituent. The preference for the pseudoaxial orientation of the phenyl substituent in rac-2 gives rise to a single diastereomer in the Grignard reaction due to the steric hindrance imposed by the phenyl substituent on one face of the carbonyl group.
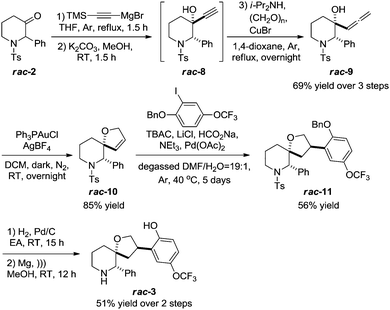 | ||
| Scheme 3 Synthesis of rac-3 using a Searles–Crabbé homologation, Au-catalyzed cycloisomerisation and reductive Heck reaction. | ||
Au-catalyzed cycloisomerisation15 of rac-9 constructed the spirocycle rac-10 in 85% yield and rac-10 was analyzed with single-crystal X-ray crystallography.11 A regio- and stereoselective reductive Heck reaction developed by Merck6b,c transformed rac-10 to rac-11 in 56% yield. The stereoselectivity of the reductive Heck reaction can be rationalized by the preferential approach of the arylpalladium species from the less hindered face of the dihydrofuran moiety in rac-10, while the regioselectivity arises due to steric considerations.16
The relative stereochemistry of rac-11 was also confirmed using NOE analysis17 where the two benzylic protons in rac-11 were shown to have NOE correlations, in agreement with the results reported by Merck. Rac-11 was finally subjected to Pd-catalyzed hydrogenation and Mg-promoted tosyl deprotection with the help of sonication to complete the synthesis of rac-3 in 51% yield over 2 steps as a white solid instead of a pale yellow oil reported by Merck6b for the reported enantiopure form. The single-crystal X-ray crystal11 structure of rac-3 is shown (Fig. 4).
We have demonstrated the efficiency of synthesizing tosyl-protected (2S)-phenyl-3-piperidone 2, a common intermediate for many drugs, from rice straw-derived furfural 1 by employing Hayashi's highly enantioselective rhodium-catalyzed arylation methodology as well as the aza-Achmatowicz rearrangement reaction. This synthetic strategy has the advantage of being a shorter route with only a single silica gel chromatography step, generating product in higher yield with almost no loss of optical purity, and originating from a renewable source, thus improving its sustainability and alleviating the problems caused by the open-field burning of rice straw. Most importantly, 2 allows facile access to numerous biologically active compounds and its synthetic usefulness has been demonstrated with the synthesis of rac-3.
This research was supported financially by Nanyang Technological University (New Initiative Funding) and the National Environment Agency (NEA-ETRP Project Ref. No. 1002 111). The authors would like to thank Dr Ganguly (NTU) and Dr Li (NTU) for their assistance with the single-crystal X-ray crystallography.
Notes and references
- (a) E. E. Hood, P. Nelson and R. Powell, Plant Biomass Conversion, Wiley-Blackwell, Chichester, 2011 Search PubMed; (b) G. Centi and R. A. v. Santen, Catalysis for renewables: from feedstock to energy production, Wiley-VCH, Weinheim, 2007 Search PubMed; (c) D. L. Klass, Biomass for renewable energy, fuels, and chemicals, Academic Press, San Diego, 1998 Search PubMed.
- (a) K. J. Zeitsch, The Chemistry and Technology of Furfural and its Many By-Products, Sugar Series, Elsevier, Amsterdam, 2000, vol. 13 Search PubMed; (b) A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed; (c) R. Xing, W. Qi and G. W. Huber, Energy Environ. Sci., 2011, 4, 2193–2205 RSC.
- (a) J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC; (b) J.-P. Lange, E. van der Heide, J. van Buijtenen and R. Price, ChemSusChem, 2012, 5, 150–166 CrossRef CAS PubMed; (c) F. W. Lichtenthaler and S. Peters, C. R. Chim., 2004, 7, 65–90 CrossRef CAS PubMed; (d) P. Srihari and Y. Sridhar, Eur. J. Org. Chem., 2011, 6690–6697 CrossRef CAS; (e) C. A. Leverett, M. P. Cassidy and A. Padwa, J. Org. Chem., 2006, 71, 8591–8601 CrossRef CAS PubMed; (f) M. P. Cassidy and A. Padwa, Org. Lett., 2004, 6, 4029–4031 CrossRef CAS PubMed; (g) J. M. Harris and A. Padwa, J. Org. Chem., 2003, 68, 4371–4381 CrossRef CAS PubMed; (h) J. M. Harris and A. Padwa, Org. Lett., 2002, 4, 2029–2031 CrossRef CAS PubMed; (i) M. H. Haukaas and G. A. O'Doherty, Org. Lett., 2001, 3, 401–404 CrossRef CAS; (j) A. Kabro, E. C. Escudero-Adán, V. V. Grushin and P. W. N. M. van Leeuwen, Org. Lett., 2012, 14, 4014–4017 CrossRef CAS PubMed.
- (a) K. L. Kadam, L. H. Forrest and W. A. Jacobson, Biomass Bioenergy, 2000, 18, 369–389 CrossRef CAS; (b) Q. Li, S. Hu, D. Chen and B. Zhu, Biomass Bioenergy, 2012, 47, 277–288 CrossRef PubMed; (c) B. Gadde, C. Menke and R. Wassmann, Biomass Bioenergy, 2009, 33, 1532–1546 CrossRef CAS PubMed.
- See ESI† for a complete list of references for the NK1 receptor antagonists.
- (a) P. E. Maligres, M. M. Waters, J. Lee, R. A. Reamer, D. Askin, M. S. Ashwood and M. Cameron, J. Org. Chem., 2002, 67, 1093–1101 CrossRef CAS PubMed; (b) D. J. Wallace, J. M. Goodman, D. J. Kennedy, A. J. Davies, C. J. Cowden, M. S. Ashwood, I. F. Cottrell, U.-H. Dolling and P. J. Reider, Org. Lett., 2001, 3, 671–674 CrossRef CAS PubMed; (c) J. J. Kulagowski, N. R. Curtis, C. J. Swain and B. J. Williams, Org. Lett., 2001, 3, 667–670 CrossRef CAS PubMed.
- (a) J. Lee, T. Hoang, S. Lewis, S. A. Weissman, D. Askin, R. P. Volante and P. J. Reider, Tetrahedron Lett., 2001, 42, 6223–6225 CrossRef CAS; (b) C. G. Kokotos and V. K. Aggarwal, Chem. Commun., 2006, 2156–2158 RSC; (c) X. Gaucher, M. Jida and J. Ollivier, Synlett, 2009, 3320–3322 CAS; (d) N. M. Garrido, M. García, M. R. Sánchez, D. Díez and J. G. Urones, Synlett, 2010, 387–390 CrossRef CAS PubMed; (e) M. Atobe, N. Yamazaki and C. Kibayashi, J. Org. Chem., 2004, 69, 5595–5607 CrossRef CAS PubMed; (f) O. Calvez and N. Langlois, Tetrahedron Lett., 1999, 40, 7099–7100 CrossRef CAS; (g) K. Takahashi, H. Nakano and R. Fujita, Tetrahedron Lett., 2005, 46, 8927–8930 CrossRef CAS PubMed; (h) P.-Q. Huang, L.-X. Liu, B.-G. Wei and Y.-P. Ruan, Org. Lett., 2003, 5, 1927–1929 CrossRef CAS PubMed; (i) L.-X. Liu, Y.-P. Ruan, Z.-Q. Guo and P.-Q. Huang, J. Org. Chem., 2004, 69, 6001–6009 CrossRef CAS PubMed; (j) S. V. Pansare and E. K. Paul, Org. Biomol. Chem., 2012, 10, 2119–2125 RSC.
- R. Adams and V. Voorhees, Org. Synth., 1941, 1, 280 Search PubMed.
- (a) N. Tokunaga, Y. Otomaru, K. Okamoto, K. Ueyama, R. Shintani and T. Hayashi, J. Am. Chem. Soc., 2004, 126, 13584–13585 CrossRef CAS PubMed; (b) G. Berthon and T. Hayashi, Rhodium- and Palladium-Catalyzed Asymmetric Conjugate Additions, in Catalytic asymmetric conjugate reactions, ed. A. Córdova, Wiley-VCH, Weinheim, Germany, 2010, pp. 1–44 Search PubMed.
- (a) O. Achmatowicz Jr, P. Bukowski, B. Szechner, Z. Zwierzchowska and A. Zamojski, Tetrahedron, 1971, 27, 1973–1996 CrossRef; (b) E. A. Couladouros and M. P. Georgiadis, J. Org. Chem., 1986, 51, 2725–2727 CrossRef CAS; (c) Review: M. A. Ciufolini, C. Y. W. Hermann, Q. Dong, T. Shimizu, S. Swaminathan and N. Xi, Synlett, 1998, 105–114 CrossRef CAS PubMed; (d) Y. Lefebvre, Tetrahedron Lett., 1972, 13, 133–136 CrossRef; (e) Z.-H. Lu and W.-S. Zhou, J. Chem. Soc., Perkin Trans. 1, 1993, 593–596 RSC; (f) Z. Wei-Shan, X. Wen-Ge, L. Zhi-Hui and P. Xin-Fu, Tetrahedron Lett., 1995, 36, 1291–1294 CrossRef; (g) W.-S. Zhou, W.-G. Xie, Z.-H. Lu and X.-F. Pan, J. Chem. Soc., Perkin Trans. 1, 1995, 2599–2604 RSC; (h) L.-X. Liao, Z.-M. Wang, H.-X. Zhang and W.-S. Zhou, Tetrahedron: Asymmetry, 1999, 10, 3649–3657 CrossRef CAS; (i) W.-S. Zhou, Z.-H. Lu, Y.-M. Xu, L.-X. Liao and Z.-M. Wang, Tetrahedron, 1999, 55, 11959–11983 CrossRef CAS; (j) H.-J. Altenbach and R. Wischnat, Tetrahedron Lett., 1995, 36, 4983–4984 CrossRef CAS; (k) J. C. P. Hopman, E. van den Berg, L. O. Ollero, H. Hiemstra and W. Nico Speckamp, Tetrahedron Lett., 1995, 36, 4315–4318 CrossRef CAS; (l) The mechanism for aza-Achmatowicz rearrangement is illustrated in the ESI†.
- The CIF files, ORTEP drawings and CCDC deposition numbers of the crystalline compounds are provided in the ESI†.
- (a) J. D. Brown, M. A. Foley and D. L. Comins, J. Am. Chem. Soc., 1988, 110, 7445–7447 CrossRef CAS; (b) S.-e. Yoo and S. H. Lee, J. Org. Chem., 1994, 59, 6968–6972 CrossRef CAS.
- M. D. Lewis, J. K. Cha and Y. Kishi, J. Am. Chem. Soc., 1982, 104, 4976–4978 CrossRef CAS.
- S. Searles, Y. Li, B. Nassim, M.-T. R. Lopes, P. T. Tran and P. Crabbé, J. Chem. Soc., Perkin Trans. 1, 1984, 747–751 RSC.
- B. Gockel and N. Krause, Org. Lett., 2006, 8, 4485–4488 CrossRef CAS PubMed.
- R. F. Heck, Palladium-Catalyzed Vinylation of Organic Halides, in Organic Reactions, ed. W. G. Dauben, John Wiley & Sons Inc, New York, 1982, vol. 27, p. 345 Search PubMed.
- COSY, HMQC and nuclear Overhauser effect analyses are available in the ESI†.
Footnote |
| † Electronic supplementary information (ESI) available: Additional text with full experimental details, characterization and crystallographic data, chromatograms and NMR spectra. CCDC 917485–917489. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc02645d |
| This journal is © The Royal Society of Chemistry 2014 |