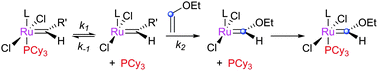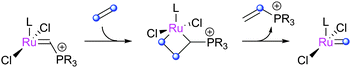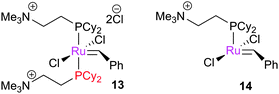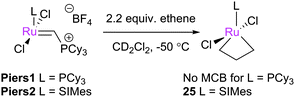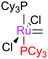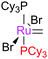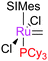Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Key processes in ruthenium-catalysed olefin metathesis
David J.
Nelson†
,
Simone
Manzini
,
César A.
Urbina-Blanco‡
and
Steven P.
Nolan
*
EaStCHEM School of Chemistry, University of St Andrews, North Haugh, St Andrews, Fife, KY16 9ST, UK. E-mail: snolan@st-andrews.ac.uk
First published on 16th June 2014
Abstract
While the fundamental series of [2+2]cycloadditions and retro[2+2]cycloadditions that make up the pathways of ruthenium-catalysed metathesis reactions is well-established, the exploration of mechanistic aspects of alkene metathesis continues. In this Feature Article, modern mechanistic studies of the alkene metathesis reaction, catalysed by well-defined ruthenium complexes, are discussed. Broadly, these concern the processes of pre-catalyst initiation, propagation and decomposition, which all have a considerable impact on the overall efficiency of metathesis reactions.
Introduction
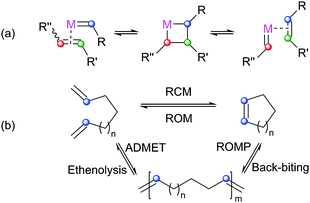
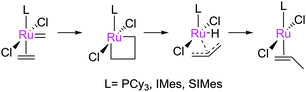
The metathesis reaction catalysed by well-defined homogeneous transition metal complexes has become a staple technique for the synthesis of a number of molecules. This progress was recognised in 2005 with the award of the Nobel Prize in Chemistry to Yves Chauvin, Robert Grubbs and Richard Schrock, for their work in this area.1–3 Metathesis reactions proceed via carbene exchange between a metal carbene and an alkene (Scheme 1(a)); a metallacyclobutane (MCB) is the intermediate species. Astruc and Lloyd-Jones have discussed early work on elucidating this basic mechanism.4,5 This basic series of steps can be used to design a variety of processes, such as: ring-closing metathesis (RCM) in which a diene substrate forms a cycloalkene plus an alkene; cross-metathesis (CM) in which two alkenes are used to prepare two new alkenes; ring-opening metathesis polymerisation (ROMP) where a cyclic alkene is used to prepare a polymer; and acyclic diene metathesis (ADMET) where a diene substrate is polymerised to form a poly(alkene) chain (Scheme 1(b)). A wide range of pre-catalysts are known, the majority of which bear N-heterocyclic carbene (NHC) ligands,6–8 although the basic mechanism is the same in each case.9 Metathesis pre-catalysts are known that bear various ancillary ligands, alkylidenes, and halides (e.g.Fig. 1).6,7,10–19 Metathesis pre-catalysts typically feature a so-called "throw-away" ligand which is not present in the active catalyst; this is typically a chelating alkoxystyrene group or a phosphine, although a wide range of such ligands have been employed.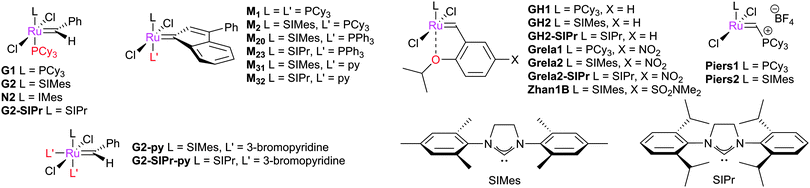 | ||
| Fig. 1 Common metathesis pre-catalysts; G indicates Grubbs-type; N, Nolan, M, Unmicore ‘M’ series indenylidene complexes; GH2, Grubbs–Hoveyda type. | ||
Work continues in this field, to elucidate the finer mechanistic details. Here, we consider the developments that are of most relevance to the ruthenium-catalysed homogeneous alkene metathesis reaction, for which a more detailed mechanistic outline can be found in Scheme 2. Key processes include: the study of pre-catalyst initiation, during which a stable pre-catalyst (typically 16e− RuII) becomes an active 14e− species; the study of how the ancillary ligand affects reactivity; the partitioning between intra- and inter-molecular metathesis pathways; the study and understanding of the key steps that occur during metathesis reactions, the development of Z-selective metathesis pre-catalysts; and the study of catalyst decomposition. Tandem catalysis (involving a metathesis step and a subsequent reaction using the same charge of ruthenium) and deleterious side reactions such as unwanted isomerisation are beyond the scope of this Review and are mentioned only briefly. While experimental studies are the main focus, a selected number of salient examples of computational studies will be discussed. Aspects of the latter topic were reviewed recently by Cavallo et al.20 and by van Sittert et al.21
Pre-catalyst initiation
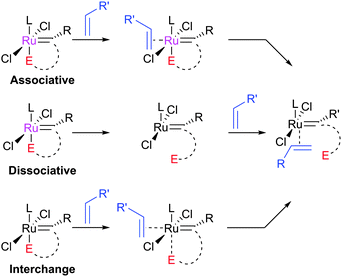
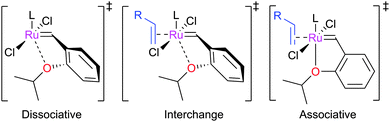
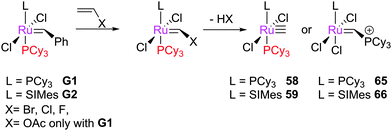
The initiation rate of a pre-catalyst determines the rate at which active 14e− species are generated; this factor has a significant impact on the overall reaction. Most pre-catalysts are 16e− species which must first lose a ligand to generate a (typically unobservable) 14e− alkylidene. This process has been studied heavily, particularly in recent years. Notably, the use of well-defined catalysts rather than ill-defined salts or heterogeneous catalysts has allowed the key steps during initiation to be studied in some detail. Initiation mechanisms are considered here for several selected pre-catalyst types; for 16e− pre-catalysts the initiation can be considered to be one of three types: associative, dissociative, or interchange (Scheme 3). In the former, alkene binds the metal centre to yield an 18e− intermediate before loss of a ligand; in the dissociative mechanism, a 14e− species is first formed that binds alkene; and in the interchange mechanism the binding of alkene and loss of a ligand occur simultaneously.Phosphine-containing pre-catalysts (Grubbs-type)
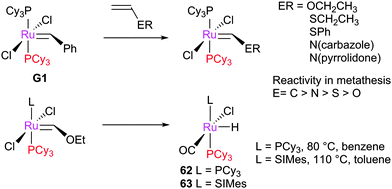
Initially, the metathesis reaction catalysed by G1 was believed to occur via an associative mechanism, via an intermediate such as 1.22 Phosphine dissociation was proposed to then allow MCB formation subsequently. Highly σ-donating phosphines were proposed to be best able to stabilise the MCB. Later work established that the first step in the mechanism was in fact phosphine dissociation (i.e. a dissociative process).23 Two methods were used to probe this process: (i) 31P NMR spectroscopy, to probe the rate of exchange of free phosphine with bound phosphine, and (ii) the reaction of the pre-catalysts with ethyl vinyl ether (EVE), for which phosphine dissociation was shown to be rate limiting (Scheme 4). Interestingly, G2 initiated much slower than G1, despite the much greater σ-donating ability of SIMes versus PCy3.24 Instead, the origin of the higher rate of activity of second generation complexes was found to be due to the preference of second generation alkylidenes for alkene over phosphine (vide infra). Initiation rates have been collected for a range of phosphine-bearing benzylidene complexes, with less electron-donating phosphines dissociating more readily.25,26 Initiation rates for G2 are known in a number of solvents,27 including some fluorinated aromatic solvents that have been proposed to enhance reactivity.28,29 Notably, changes to the dissociating phosphine ligand do not change the nature of the active species; the same 14e− alkylidene is generated upon initiation.The differences in initiation between first- and second-generation pre-catalysts have intrigued many chemists. Initially, it was thought that the higher trans-effect of the NHC versus the phosphine should render the activation of G2 faster than that of G1. However, Kennepohl has shown, using Ru K-edge X-ray absorption spectroscopy, that the metal centre of G2 is in fact more electron deficient than in G1 due to d to π* back-bonding in G2.30 DFT studies have sought to reproduce the experimental initiation data. Some key studies deserve special mention: Truhlar has shown the importance of dispersive interactions in calculating initiation rates;31 Goddard has successfully reproduced experimental energies, but required the use of an explicit solvent molecule to occupy the vacant site on the product 14e alkylidene;32 Jensen has carried out detailed calculations for a number of complexes, achieving good agreement with experiment using counterpoise- and dispersion-corrected B3LYP calculations;33 and Truhlar has also proposed that carbene rotamer switching is the underlying cause of the intriguing initiation rate differences between G1 and G2.34
Nolan has studied the initiation of indenylidene species such as M20 and M23, using [31P, 31P] EXSY and EVE quench experiments.35 The indenylidene moiety was shown to lead to a decrease in initiation rate compared to the analogous benzylidene complexes. However, most interestingly, M20 was shown to initiate via an interchange mechanism rather than a dissociative mechanism; i.e. alkene binding and phosphine dissociation occur during one concerted step. Activation parameters showed a negative entropy of activation (ΔS‡ = −13 ± 8 cal K−1 mol−1) for M20versus a positive entropy of activation for complexes such as M1 (ΔS‡ = 8 ± 4 cal K−1 mol−1), G2 (ΔS‡ = 12 ± 10 cal K−1 mol−1), and M23 (ΔS‡ = 21 ± 3 cal K−1 mol−1). DFT calculations were performed to support this work, which indicated a rather fine balance between dissociative and interchange mechanisms, with typically only a few kcal mol−1 difference between the two pathways.
A table of activation rates for selected phosphine-containing pre-catalysts can be found in Table 1, illustrating how these span a considerable range.
| Complex | k init | ΔH‡ (kcal mol−1) | ΔS‡ (cal K−1 mol−1) | ΔG‡ (kcal mol−1) |
|---|---|---|---|---|
| G1 | 9.6(2) s−1 | 23.6(5) | 12(2) | 19.88(6) |
| G2 | 0.13(1) s−1 | 27(2) | 13(6) | 23.0(4) |
| M1 | 1.72 s−1 | 23(1) | 8(4) | 21(2) |
| M20 | 0.19 L mol−1 s−1 | 17(3) | −13(8) | 21(4) |
Chelating benzylidene-ether pre-catalysts (Hoveyda-type)
Initially, Hoveyda-type complexes were proposed to initiate via a dissociative mechanism, where the transition state (TS) involves partial rotation of the alkylidene and scission of the Ru–O bond (Fig. 2).26 Later work showed that the entropy of activation was actually negative36 (ΔS‡ for GH2 = −19 ± 3 cal K−1 mol−1), consistent with either an interchange mechanism, where Ru–O scission occurs in tandem with the approach of the alkene substrate, or the associative mechanism where a six-coordinate intermediate forms before Ru–O bond scission. The first detailed study of the initiation mechanism of this class of catalysts was carried out by Plenio, who studied the initiation of GH2 and Grela2 using UV/visible spectroscopy.37 Kinetic experiments showed that the initiation rates of GH2 and Grela2 depended on the identity and concentration of the alkene substrate. Subsequently, Percy and Hillier studied the initiation of GH2 using kinetic experiments and DFT calculations.38 Experimental and theoretical activation parameters for this complex were in agreement. The dissociative, associative and interchange mechanisms were all studied in silico, with the latter presenting the lowest barrier.Following this, Plenio and co-workers published a detailed study of a range of complexes and substrates.39 A mixture of dissociative and interchange mechanisms was proposed to operate, with the balance of these processes dependent on the pre-catalyst and substrate. Much of this was based on the observed curvature of a plot of kobsversus [substrate].
In a detailed computational study, Solans-Monfort and co-workers modelled the initiation of some Hoveyda-type complexes with model substrates.40 Notably, they pointed out that the choice of starting point can affect the outcome: if a pre-reactant complex is considered, the entropic cost of associative/interchange mechanisms is underestimated; if not, it may be overestimated. The authors ruled out the associative mechanism, but could not firmly distinguish between the interchange and dissociative mechanisms. The final dissociation of the η2-vinylaryl moiety was also proposed to have a large barrier, which may affect the overall rate.
Initiation rates for a number of Hoveyda-type complexes have been published.39,41,42 The SIPr ligand significantly decreases the initiation rate of these complexes, in contrast to the trend observed with Grubbs-type species;42GH2-SIPr initiates ca. 10-fold slower than GH2. In addition, initiation rates for GH2 have been recorded in a range of solvents.27
A later computational study by Hillier, Percy, and co-workers evaluated the potential energy surfaces (PES) for the initiation of three complexes with a selection of substrates.43 The metathesis of ethene was found (both experimentally and computationally) to be energetically unfavourable overall, while the metathesis of EVE was highly favourable. Notably, the barriers on the PES for metallacyclobutanation are as high as those for the initial interchange step, suggesting that more than one step in the sequence has a considerable influence on the overall rate.
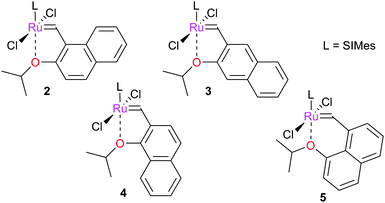
Naphthalene-based Hoveyda-type complexes have been explored in metathesis; studies of complexes 2–4 suggest that the degree of aromaticity is important in determining initiation rate.44 Barbasiewicz et al. later synthesised the corresponding peri-substituted naphthyl-based complex 5, which was shown to be a more rapid initiator than other naphthyl-based systems.45
A table of activation rates for selected Hoveyda-type pre-catalysts can be found below (Table 2).38,41–43 Electron-withdrawing or bulky substituents accelerate the initiation, compared to electron-donating groups.
Vinylphosphonium complexes (Piers-type)
These species17 initiate rapidly, which has led to their use for interesting mechanistic studies that would be very difficult to accomplish otherwise.46–53 The basic initiation mechanism involves a [2+2]cycloaddition with ethene, which releases a vinylphosphonium byproduct and generates the 14e− methylidene rapidly (Scheme 5).49 The byproduct was shown to be a Type IV olefin54 (vide infra) which does not undergo metathesis. Piers-type catalysts smoothly form Hoveyda-type complexes if exposed to the corresponding alkoxystyrene. Competing dimerisation can occur (vide infra), but this can be mitigated by the appropriate phosphonium substitution pattern, with triisopropylphosphine-based complexes highlighted as striking the best balance between stability and reactivity.Phosphite complexes (Cazin-type)
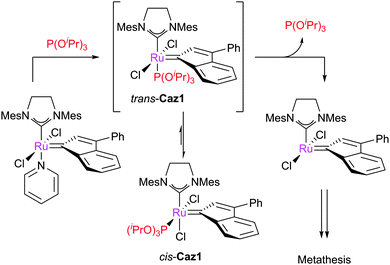
Cazin and co-workers have recently prepared and studied a series of NHC–phosphite complexes. Phosphites are more π-acidic, and have different electronic and steric properties, compared to phosphines.55 Cazin reported the synthesis of Caz1via simple ligand displacement (Scheme 6).56,57Surprisingly, the product complex featured cis-chloride rather than the expected trans-chloride geometry. Analogous benzylidene complexes exhibit the expected trans-geometry.58 For SIPr-bearing indenylidene species, the use of P(OEt)3 leads to the trans-chloride complex, while for P(OiPr)3 only the cis-species can be accessed.59Caz-1 must undergo a cis-trans isomerisation before yielding an active 14e− species by ligand dissociation. The barrier is considerable (ΔH‡ = 22.6 kcal mol−1; ΔS‡ = −4.2 cal K−1 mol−1),56 and so the catalyst is only active at high temperatures, but exhibits excellent stability under ambient conditions. The addition of excess phosphite did not affect the rate of isomerisation, suggesting that ligand dissociation/re-association was not involved.
Heteroatom-chelated complexes
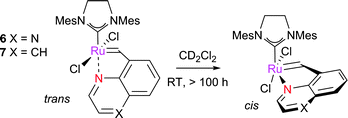
Latent complexes, typically bearing chelating nitrogen- or sulfur-based functionality, have also attracted attention.60,61 High stability and low activity under ambient conditions allow for easy handling, before activation using a specific stimulus such as light, heat, or an additive. Some studies have been conducted on the behaviour of some of these complexes, to investigate parameters relevant to their initiation, mainly around their cis–trans isomerisation. This issue is relevant to initiation, as these two isomers will exhibit different initiation behaviours.Quinoline complexes such as 6 and 7 exist in either cis- or trans-geometries, which interconvert when heated (Scheme 7).62 The trans-isomer is the most active in catalysis, although reactivity at room temperature was much poorer than common pre-catalysts such as G2. DFT studies suggested that the pathway in which the nitrogen remained bound to the ruthenium during this isomerisation was lowest in energy; however, there was a small difference between this pathway and the one in which the Ru–N bond is disrupted and re-formed.63 Sulfur- and halide-chelated complexes also exhibit isomerisation from the trans- to the cis-configuration,64,65 although such processes have not been studied in as much detail for these systems.66 For some complexes, the intermediate trans-isomer is not observable.
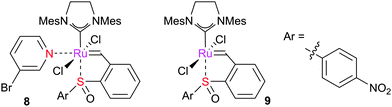
Grela et al. studied the initiation of six co-ordinate complexes such as 8;67 these were found to be more reactive than the parent complexes (e.g.9). DFT studies revealed that this was the result of a different initiation mechanism, where the pyridine ligand assisted debinding of the sulfoxide and thus co-ordination of the alkene substrate.
Pyridine-bearing complexes
The study of rapidly initiating pyridine-bearing complexes has been explored recently. Initially, only an estimate of initiation rate for species such as G2-py was available.13 Trzaskowski and Grela recently investigated the initiation of complexes of this type using DFT calculations.68 Three mechanisms were considered: one where the (bromo)pyridine ligands dissociate before alkene binding, one where alkene binding occurs with a (bromo)pyridine ligand attached (via an intermediate six coordinate complex), and one where the second (bromo)pyridine ligand is displaced by substrate in an interchange fashion. The authors concluded that the dissociative mechanism was favoured, although for small substrates the associative mechanism was plausible.Metathesis propagation
While the initiation event is critical in determining how quickly 14e− active species are formed, the actions and activities of these species also determine the overall efficiency of the reaction.First- versus second-generation pre-catalysts
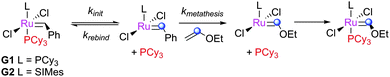
Since the introduction of the first NHC-bearing complexes, significant differences in reactivity have been apparent between these second generation complexes and the bis(phosphine) first generation pre-catalysts. While early second generation species, such as Herrmann's bis(NHC) complexes69 performed poorly, it was realised quickly that heteroleptic NHC–phosphine12,19,70 and NHC–chelating ether15 complexes showed better reactivity. As discussed above, this reactivity difference was despite the much slower initiation of second generation complexes, as discovered by Grubbs and co-workers,23 who conducted a series of experiments to explore the differences between first- and second-generation metathesis complexes.25 When these complexes were exposed to defined ratios of EVE and phosphine, reversible phosphine binding could compete with the (effectively irreversible)71 reaction with EVE (Scheme 8). The observed pseudo-first order rate constants for the decrease of pre-catalyst concentration plotted versus [phosphine]/[EVE] furnished a straight line with intercept 1/kinit and gradient krebind/(kinit·kmetathesis) (eqn (1); Fig. 3). G2 is equally selective for phosphine and alkene, while G1 is 103 times more selective for phosphine. While G1 initiates more rapidly, it is less likely to undergo productive metathesis before being trapped by phosphine; the initiation rates alone do not determine the overall reactivity, as one must consider the activity and selectivity of the 14e− species also.| 1/kobs = (krebind·[PCy3])/(kinit·kmetathesis·[EVE]) + 1/kinit | (1) |
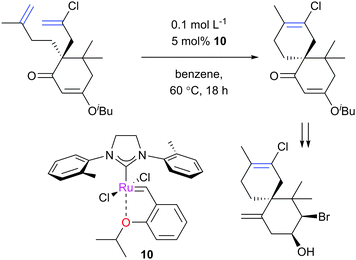
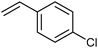
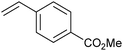
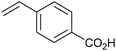
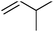
As well as this general difference in behaviour towards alkenes, first- and second-generation catalyst systems will react differently with some substitution patterns found in substrates. Grubbs et al. developed a useful system of classification for alkene termini, predominantly for use in predicting or rationalising CM reaction outcomes.54 Each alkene structural motif can be categorised as Type I–IV. Type I alkenes react (and dimerise) quickly, but dimers are consumable; a number of alkenes have been charged to CM reactions as the dimer. Type II alkenes react (and dimerise) more slowly, and the corresponding dimers are consumed slowly. Type III alkenes are still reactive in CM, but will not dimerise. Type IV alkenes do not react, but will not poison catalysts, and therefore act as ‘spectators’. Categorising each CM partner for a given reaction allows the outcome to be predicted. The category into which a given motif falls is a function of the pre-catalyst, with different behaviour observed for molybdenum complexes, and for first- and second-generation ruthenium complexes. Notably, the reactivity of second-generation complexes varies considerably, depending on the nature of the dissociating ligand and on the identity of the NHC ligand. For example, complexes bearing less hindered NHCs have been utilised to prepare heavily-substituted alkenes, such as in Stoltz's synthesis of (+)-elatol, where complex 10 enabled the preparation of the challenging tetrasubstituted cyclohexene unit (Scheme 9).72
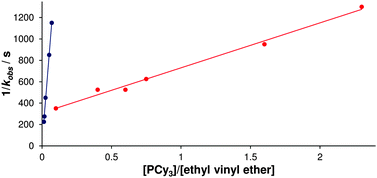 | ||
| Fig. 3 Plot of 1/kobsversus [PCy3]/[ethyl vinyl ether] for the metathesis of EVE with G1 (blue) and G2 (red) in the presence of added PCy3.25 | ||
For this reason, there is no universal ‘best’ catalyst,73 and research is currently underway in a number of laboratories to develop new catalysts and apply these to otherwise challenging or impossible substrates and reaction conditions.
The degree of thermodynamic control in a metathesis reaction will also depend on the pre-catalyst system; the issue of thermodynamic control has been reviewed recently by Fogg et al.74 Second-generation catalyst systems can react with starting materials (i.e. typically monosubstituted alkenes) and products (typically 1,2-disubstituted alkenes),54 so can more easily approach the thermodynamic end point of a reaction. This was demonstrated experimentally by Percy and co-workers, who showed that the RCM of 1,8-nonadiene (using different loadings of G2) and the ROMP of cycloheptene (in the presence of ethene) led to the same equilibrium mixture.75 It was also evident when Grubbs et al. explored the development of a system for pre-catalyst characterisation, using a number of model reactions.76 In a prototypical benchmark reaction (Scheme 10), second generation catalysts achieved better E/Z selectivities in shorter periods of time than first generation species (Fig. 4). The E/Z selectivity of a metathesis reaction is a key outcome that will affect the viability of a process. While RCM to form smaller (ca. 5–8 membered) rings almost77 always produces Z-alkenes, CM reactions and RCM reactions to produce larger rings (such as macrocycles)78 can yield the E- or Z-isomer. A fuller discussion of E/Z selectivity can be found in a subsequent section of this manuscript.
 | ||
| Scheme 10 A prototypical cross-metathesis reaction.76 | ||
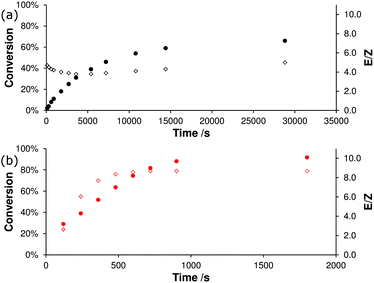 | ||
| Fig. 4 Conversion to product (closed circles) and E/Z ratio (open diamonds) versus time for the reaction in Scheme 10 with (a) 2.5 mol% G1 or (b) 2.5 mol% of G2.76 | ||
Several studies have sought to explain the reactivity differences between first- and second-generation catalysts. Cavallo explored the early stages of metathesis with a series of complexes, from initiation to MCB formation, in one of the first DFT studies of metathesis that considered untruncated structures (Scheme 11).79 Calculations on the MCB formation step showed that second generation complexes (N2 and G2, bearing IMes and SIMes, respectively) face lower barriers than first generation G1, and yield MCBs that are lower in energy. In addition, the steric pressure exerted by the (S)IMes ligands destabilised the phosphine-bound and olefin-bound intermediates, leading to more energetically favourable metathesis but not increased initiation rate.
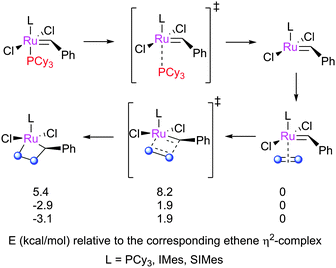 | ||
| Scheme 11 Structures explored by Cavallo.79 | ||
Straub conducted calculations on methylidene complexes 11 and 12, and the four conformers of their η2-complexes with ethene.80 In the reactive conformer, the alkene and the carbene are in the same plane, and aligned in the same direction (Fig. 5). For second-generation complexes, the energy difference between reactive and unreactive conformers was smaller than for first-generation complexes, explaining the observed reactivity differences. Attempts to optimise the structure of the reactive conformer led to MCB formation. A later study evaluated the bonding in active ruthenium carbene complexes to rationalise the effect of the NHC on their stabilisation.81 In common with other DFT studies (vide infra), it was shown that second-generation MCBs are relatively low in energy, as opposed to the first-generation MCBs which are relatively high in energy and have not been observed spectroscopically.46 The pathway proceeding via cis-dichloride intermediates (i.e. via side-bound MCBs) was shown to be far less favourable.
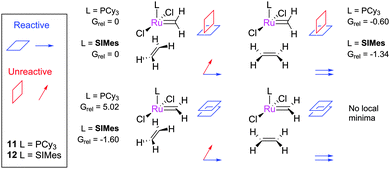 | ||
| Fig. 5 The four conformers of the complex between methylidene and ethene; relative energies are in kcal mol−1 using the B3LYP density functional.80 | ||
Lavigne et al. proposed that through space π–π* interactions between the aryl moiety on the NHC and the ruthenium carbene stabilise NHC-bearing complexes.82 Plenio and co-workers used electrochemical measurements to show their existence.83,84 Cavallo and co-workers have shown that, for transition metal complexes in general, there exist interactions between the aryl rings of bis(aryl)-NHCs and the transition metal d-orbitals.85 The electron density of these aryl rings can therefore modulate this interaction. This is, of course, an interaction that is not available in first generation complexes.
Metathesis catalysts can react with alkynes and allenes, as well as alkenes, allowing elaborate cascade reactions. Sohn and Ihee used time-dependent fluorescence quenching studies to explore the affinity of metathesis catalysts for alkenes, allenes and alkynes. During enyne metathesis, G1 favoured reaction with the alkene terminus first.86 In a subsequent study, molybdenum complexes were shown to favour alkyne over alkene over allene, while first generation ruthenium complexes favoured allene over alkene over alkyne.87 Second-generation ruthenium complexes favoured alkyne over allene over alkene. Understanding this order of selectivity is important in the design of cascade metathesis reactions.
The use of NHCs as ligands for metathesis pre-catalysts has allowed access to a vast range of interesting catalyst structural motifs, each with different reactivity.6,7 Such ligands can be prepared using a variety of established and scalable organic synthetic chemistry methodology.88 While monodentate phosphine ligands sterically influence the metal centre in a limited number of ways (measured using the concept of ‘cone angle’),55 NHCs can be constructed from various organic scaffolds and can therefore influence the steric environment of the metal in various ways. These differences are best quantified using the percent buried volume (%Vbur) metric, which quantifies the percentage of the volume of a sphere (typically of 3.5 Å radius) occupied by the ligand.89 Steric maps which describe how various sections of the co-ordination sphere are occupied can also be constructed,90 using the freely-available online SambVca tool.91 The electronic properties of the range of NHCs known can also be quantified and compared.24,92
Detailed studies have therefore shown that the reactivity difference between first- and second-generation complexes lie in the metathesis steps as well as in the initiation pathways. More favourable MCB formation in particular leads to an enhancement of catalytic performance. The highly flexible NHC scaffold has allowed for a wide variety of complexes to be prepared; for example, N-alkyl-N-aryl-bearing NHCs have found application as selective ethenolysis catalysts,93 and in the preparation of small cyclic oligomers instead of long-chain polymers.94 More novel complexes with new and interesting reactivities will surely follow in years to come.
Partitioning between intra- and inter-molecular pathways
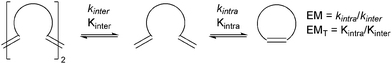
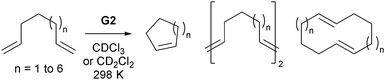
As all alkene metathesis reactions proceed via the same basic [2+2]cycloaddition mechanism, using the same functional groups, catalysed by the same active species, different processes may sometimes compete. CM reactions seek the selective coupling of two different alkene partners, and therefore the challenge lies in this selectivity. In contrast, the RCM reactions of dienes can be complicated by competing CM processes to produce dimers or polymers. An understanding of the kinetics and thermodynamics of these processes can help mitigate the impact of these deleterious reactions on RCM.While the concept of effective molarity (EM)95,96 (Scheme 12) has been applied widely in the study of acid- and base-catalysed nucleophilic ring-closing chemistry, there are relatively few examples of this concept in metathesis chemistry. Fogg et al. have probed the competition between intra- and intermolecular metathesis in some model reactions, and tabulated EM ranges for some compounds from the literature.97 Many of these ranges are broad, and are for cycloalkanes rather than cycloalkenes, which will have different EMs;98 none are for RCM. The authors also proposed that oligomeric material is an intermediate in the preparation of medium (7, 8 and 9-membered) rings. However, metathesis catalysts can be difficult to quench; careful treatment of samples is necessary to deactivate the catalyst, as concentration and analysis (by GC) while the catalyst is still active will lead to an effective increase in the reaction concentration and misleading data on the degree of conversion and oligomerisation.99 Later studies, where high resolution (600 MHz) NMR spectroscopy was employed to follow the reactions of simple prototypical α,ω-dienes with G2 (Scheme 13),100 did not detect the behaviour reported by Fogg. Instead, slow oligomerisation competed with ring-closing.
Percy et al. showed that the metathesis reactions of these simple substrates are under thermodynamic control;75 metathesis of the products yielded the same final mixture as metathesis of the substrates. Importantly, it was shown that the reaction outcomes were predictable, using thermodynamic data available in the literature98,101 or from DFT calculations (Table 3). In this manner, the optimal initial reaction concentration can be selected on the basis of straightforward calculations, rather than by expensive and time-consuming trial and error. The ratio of intra- to intermolecular products also depends on the degree of thermodynamic control. In the example above, the ring-opening of strained cycloalkenes is typically fast, allowing the reaction to reach a thermodynamic end point within hours. For reactions where product ring-opening is slow, the final ratio of cyclic product to oligomer will depend predominantly on the rate of the formation of each species. In contrast, where reactions are under thermodynamic control, the same equilibrium position will be achieved regardless of whether this position is approached from the substrate or from the product.
| Ring size | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|
| a From DFT calculations (M06-L/6-311G**) with 6 kcal mol−1 added to ΔGRCM (see ref. 75). b From an empirical treatment98 of literature data. c From a series of small scale metathesis experiments, by 1H NMR. | ||||||
| log10(EMDFTT)a | −0.78 | 1.75 | −1.25 | −3.35 | −5.90 | −3.87 |
| log10(EMDFTT)b | −0.63 | 1.52 | −2.52 | −3.31 | −6.24 | −3.48 |
| log10(EMDFTT)c | −0.27 | ≫0.60 | −1.28 | −3 to −4 | <−4 | <−4 |
The partitioning between intra- and intermolecular metathesis is therefore a function of the pre-catalyst and substrate structure. While some studies have been conducted there remains considerable scope for further understanding of these factors, and the use of this understanding in the design of new pre-catalysts and synthetic reactions.
Study and understanding of key processes and intermediates
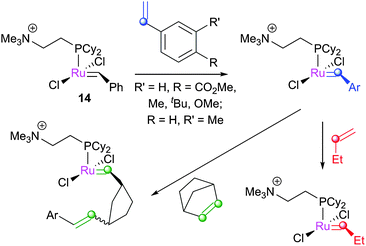
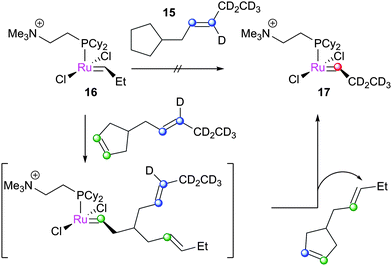
Several groups have applied ESI-MS techniques to study metathesis reactions in the gas phase. Reaction mixtures are passed into the mass spectrometer, allowing the various products and intermediates to be identified and manipulated. The Chen group have contributed a great deal in this area. In the first report, 13 (bearing ionically-tagged phosphines) underwent phosphine dissociation in the spectrometer to yield 14e− species 14.102 Such 14e− species have, despite advanced low temperature NMR studies (vide infra), never been observed in the solution phase. The reactions of 14 with substrates such as 1-butene and norbornene were probed, with strained cycloalkenes undergoing reaction more quickly: norbornene underwent reaction 15-fold faster than 1-butene, and 1500 times faster than cyclopentene. The direct detection of the 14e− species is one of the major advantages of this method, although modified species bearing ionic tags are necessary.103Subsequently, these experiments were compared to DFT calculations.104 Various complexes were prepared in the spectrometer, and subjected to reaction with 1-butene or norbornene (Scheme 14). Electron-withdrawing substituents accelerated the reaction with 1-butene (ρ = 0.69 ± 0.10). Subsequent experiments probed the reversibility of the reaction; 15 underwent ring-opening followed by ring-closing when exposed to 16, to generate 17 (Scheme 15). The corresponding cyclopentane substrate was unreactive. DFT calculations, using the highly truncated model system [RuCl2(PH3)2(CH2)] to represent [RuCl2(PCy2(CH2CH2NMe3))(CH2)]+, showed that the MCB was higher in energy than the η2-complex, and that the cis-chloride MCB complex was lower in energy than the trans-isomer, but kinetically inaccessible. PH3 is a simple phosphine, so this energy difference between isomers is likely to be different in systems with a bulky trialkylphosphine.
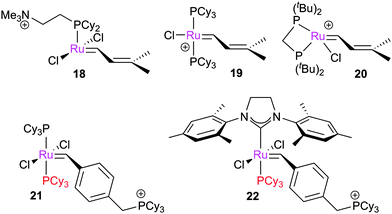
Subsequent studies explored other themes. The activity of complexes 18, 19 and 20 in the gas phase were compared, and found not to reflect those in solution; the authors suggested that this is due to the equilibrium in place before the active complex is generated, highlighting the need to understand the initiation behaviour of these complexes.105
These studies continued to develop more detailed and informative techniques. In a study of 21 and 22, bearing cationic benzylidene ligands, variation of the collision energies allowed the energetic landscape of the reaction of these complexes with norbornene to be probed.106 Excellent agreement was achieved with DFT studies,31 where the M06-L/TZP-CP level of theory was used; BP86/ZORA-TZP led to much poorer agreement.
Metzger has been active in this area, detecting the products of reactions of complex G1 with ethene and prototypical RCM substrates. Intermediates throughout the catalytic cycle were detected.107 A subsequent study quantified the relative rates of RCM of some simple dienes (see Schemes 11 and 16), where 1,7-octadiene reacted fastest, followed by 1,6-heptadiene and 1,8-nonadiene at approximately equal rates.108 Further, the equilibrium between chelated η2-complexes 23 and phosphine-bound alkylidenes 24 was probed (Scheme 16); the latter must first dissociate the phosphine before MCB formation from the chelated η2-complex can occur. Surprisingly, 1,5-hexadiene-derived complex 23a was considerably more stable than the phosphine-bound species. Later studies by Ashworth et al. established via DFT studies that the chelated complex was very stable, due to favourable interactions and a lower entropic penalty for cyclisation than longer dienes, in which more rotors must be frozen.100 1,6-Hexadiene had an inhibitory effect on the metathesis of 1,6-heptadiene and 1,7-octadiene.
The majority of studies have been conducted with first generation systems, as rapid phosphine dissociation allows quick generation of interesting intermediate carbene species. The study of alkali metal adducts of metathesis catalysts precludes the requirement for the time-consuming synthesis of modified complexes with ionic functionality. While these techniques have been applied to some studies of second generation carbene complexes,106 and most recently to studies of pre-catalyst initiation,109 there is further scope to investigate the reactivity of ruthenium carbene complexes using this approach.
A significant advance was made when Piers and co-workers developed rapidly-initiating 14e− ruthenium carbene complexes such as Piers1 and Piers2.17,18,49 While the rapid, irreversible generation of large quantities of 14e− carbenes (on reaction with ethene) renders these species non-ideal for longer reactions where decomposition may adversely affect performance, they have found application in mechanistic studies, particularly as heteroleptic NHC–phosphine complexes initiate slowly.25 This class of compound has enabled the preparation, observation and study of MCB species using modern low-temperature NMR spectroscopic techniques.46 In the first report of this reactivity, Piers demonstrated that the reaction of Piers2 with 2.2 equiv. of ethene yielded quantitative conversion to parent MCB 25 (Scheme 17). The NMR data suggested a bottom-bound MCB, while Hα exhibited a resonance at 6.6 ppm and Hβ at −2.6 ppm. 1JCH coupling constants suggested a ‘kite-shaped’ MCB. First generation analogue Piers1 yielded no observable MCB, consistent with DFT calculations that suggest that first generation MCBs are less stable than their second generation analogues.79
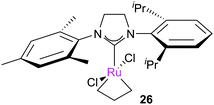
Further studies from the Piers and Grubbs groups followed. Grubbs et al. studied the reaction of Piers-type complexes with ethene and propene.51 This study established that MCBs where the two N-substituents of the NHC were different (such as 26) exhibit two resonances for Hα protons, indicating slow rotation of the NHC (i.e. on the NMR timescale). The bottom-bound nature of the MCB was also confirmed. In addition, 2D [1H, 1H] EXSY spectroscopy enabled the measurement of the rate of degenerate exchange between the α- and β-positions via MCB breakdown, ethene rotation, and MCB formation (k = 26 ± 2 s−1, corresponding to ΔG‡ = 12.2 kcal mol−1 at 233 K).
The Piers group probed the reactions of this MCB species in detail.47 Exchange with free ethene-13C2 was much slower than intramolecular α/β-exchange (k = (4.8 ± 0.3) × 10−4 L mol−1 s−1), suggesting that ethene binding or unbinding events presented significant barriers. Interestingly, determination of the thermodynamic parameters for this intermolecular reaction (ΔH‡ = 13.2 ± 0.5 kcal mol−1; ΔS‡ = −15 ± 2 cal K−1 mol−1) suggested an associative mechanism for the exchange; ethene is the smallest possible olefin, and therefore it is possible that the metal centre might be able to co-ordinate two ethene molecules.
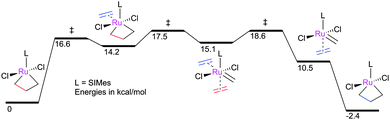
Webster has carried out calculations to probe degenerate ethene exchange.110 Initial calculations on intramolecular ethene exchange produced results consistent with the aforementioned experimental studies. Bottom-bound MCBs bearing trans-chloride ligands provided the lowest energy pathway; the calculated barrier for the exchange (ΔG‡ (DCM, 227 K) = 14.4 kcal mol−1) was in excellent agreement with the measured value. The calculated pathway for associative ethene exchange (Fig. 6) features octahedral six co-ordinate intermediates between the two MCBs with TSs for MCB formation and breakdown where twisting of the ethene ligand (to render it parallel to the Cl–Ru–Cl vector) is concomitant with C–C bond cleavage and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond formation. The involvement of a six co-ordinate metallacyclohexane was ruled out. The calculated barrier for this process (ΔG‡ (DCM, 227 K) = 18.6 kcal mol−1) was in excellent agreement with experiment (ΔG‡ (DCM, 227 K) = 16.9 kcal mol−1). These results raise questions about the involvement of high-energy four co-ordinate 14e ruthenium carbene intermediates; however, this example covers only the case of ethene, which is a particularly unhindered substrate, so more hindered substrates may behave differently.
C bond formation. The involvement of a six co-ordinate metallacyclohexane was ruled out. The calculated barrier for this process (ΔG‡ (DCM, 227 K) = 18.6 kcal mol−1) was in excellent agreement with experiment (ΔG‡ (DCM, 227 K) = 16.9 kcal mol−1). These results raise questions about the involvement of high-energy four co-ordinate 14e ruthenium carbene intermediates; however, this example covers only the case of ethene, which is a particularly unhindered substrate, so more hindered substrates may behave differently.
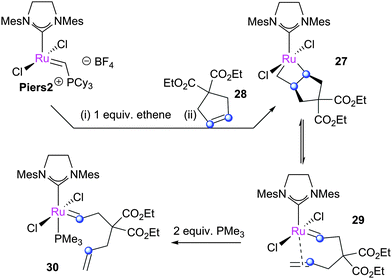
Piers and co-workers subsequently examined the ring-closing metathesis of diethyl diallylmalonate using complex Piers2, wherein complex 27 was detected spectroscopically.48 While generated from the reaction of MCB 25 with gem-disubstituted cyclopentene 28 (Scheme 18), this intermediate is formally the product MCB from the metathesis of diethyl diallylmalonate. This intermediate undergoes reversible retro[2+2]cycloaddition to yield propagating carbene species 29, which was trapped by the addition of PMe3 to yield complex 30. In addition, the ring-opening of acetonaphthalene could be achieved in the presence of ethene, forming the corresponding ruthenium carbene complex.48 Other closely related MCBs relevant to RCM chemistry could be prepared; alkenes such as cyclohexene, 3,3-dimethylbut-1-ene, and 1,1-difluoroethene did not generate the corresponding MCBs, however. The 13C chemical shifts and proposed structures were later confirmed by DFT calculations;111 the Ru–Cβ interaction was proposed to be the cause of stabilisation of the MCB, with a bond order of ca. 0.3.
Piers later published a detailed study of RCM using low-temperature NMR techniques,50 in which the PES of the reaction at 220 K was mapped by measuring key rate constants. Such detailed mapping of the PES typically requires theoretical tools rather than experimental ones, so the provision of experimentally measured energies is exciting. This work suggests that MCBs may well function as ‘protecting groups’ for the high-energy, fragile 14e ruthenium carbene complex.
A full paper was published by Grubbs in 2011,52 where Piers2 underwent reaction with propene, 1-butene and 1-hexene to yield the corresponding MCBs. The composition of the MCB mixture varied with time and temperature, which was proposed to be driven by the loss of ethene; parent MCB 25 was favoured initially, while trans and cis α,α′-disubstituted MCBs were in equilibrium (e.g. 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 31
1 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32). Surprisingly, no β-substituted MCBs were obtained, even when 1,2-disubstituted alkenes were employed, suggesting that these are higher in energy.
32). Surprisingly, no β-substituted MCBs were obtained, even when 1,2-disubstituted alkenes were employed, suggesting that these are higher in energy.
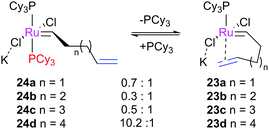
MCBs derived from unsymmetrically-substituted NHCs have been studied.53 The investigation of these complexes revealed varying rates for degenerate ethene exchange with different catalysts and, most intriguingly, that the de-binding rate of the cycloalkene was much lower than in complexes bearing, for example, SIMes. While these results were obtained at conditions far from those used in synthetic laboratories, this observation suggests that slow product de-binding may allow for more non-productive cycles per productive cycle, and therefore slower metathesis overall. Further, this observation is relevant to the proposal by Solans-Monfort and co-workers that the alkene de-binding step may play an important role in the initiation of Hoveyda-type metathesis catalysts.40
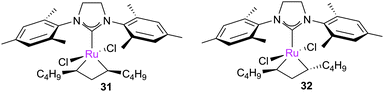
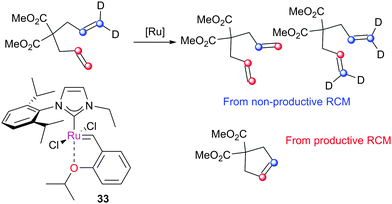
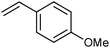
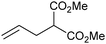
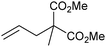
Grubbs et al. investigated the relative ratio of non-productive to productive cycles for a range of pre-catalysts in the solution phase under typical synthetic RCM conditions.112 The metathesis of diethyl diallylmalonate-d2 yields the corresponding cyclopentene from productive metathesis, while diethyl diallylmalonate-d0 and -d4 result from non-productive metathesis (Scheme 19). Common pre-catalysts such as G2 and GH2 performed 10 productive cycles per non-productive cycle, while pre-catalysts bearing unsymmetrical NHCs (e.g.33) performed as many (or more) non-productive as productive cycles. These differences were proposed to be due to the differing steric environments in these types of complexes. While many intermediates are not observable under typical experimental conditions in the solution phase, a number of elegant experiments have been utilised to probe equilibria and processes occurring in metathesis reactions.
Grubbs et al. investigated the effect of alkylidene structure on reactivity with 1-alkenes using kinetic studies of first-generation G1 and derivatives.113 Bulky alkenes were found to react slowly (or not at all); 3,3-dimethylbut-1-ene, 2-methylpent-1-ene and 1-phenylprop-1-ene were poorly reactive.
Lane et al. examined the effects of alkene structure on the formation of ruthenium carbene complexes.114 Complexes were prepared from the reaction of G1 with a range of alkenes. Equilibrium was established in each case, with a wide range of constants (Table 4; for larger 1-alkenes, Keq tends to ca. 0.3, and ΔG to ca. 0.7 kcal mol−1). Steric and electronic properties play a role, with smaller, electron rich alkylidenes being preferred. A good correlation was obtained for the free energies of reaction versus a linear combination of Hammett σr and σi parameters (ΔG = (0.90·(2.7·σr + σi) − 0.0036) kcal mol−1). For particularly bulky alkenes, such as those bearing quaternary homoallylic positions, the reaction was very unfavourable with Keq as low as 10−3. These results may explain why the RCM reaction of diethyl diallylmalonate occurs slower than that of 1,6-heptadiene,115 despite the clear thermodynamic preference of the diethyl diallylmalonate to undergo ring closing.
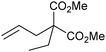
Grubbs and co-workers prepared and studied model compound 34, in conformations a and b.116 Surprisingly, trans-34 was not observed; crystalline material obtained was composed of cis-34a, which provided cis-34b on heating. This process was under thermodynamic control, with higher temperature favouring cis-34b. Detailed NMR experiments confirmed the assignments. Piers and co-workers have characterised a related example from the ring-opening of acenaphthylene.48 These are particularly rigid examples of chelated alkylidenes; Snapper and co-workers have previously isolated and characterised trans-dichloride chelated alkylidene 35, based on a 1,2-divinylcyclobutane motif;117 the structural effects which decide if cis- or trans-isomers are favoured in solution are therefore likely to be somewhat subtle.
A subsequent detailed study evaluated more complexes.118 The dynamic solution behaviour varied; for 36, no exchange between the two cis-isomers occurred at room temperature. The SIPr complex showed strong preference for the isomer in which the terminal CH2 is directed towards the NHC (analogous to 37), while chiral complexes such as 38 did not undergo exchange. Complexes bearing PCy3 in place of an NHC underwent exchange. When a bulkier olefin, 1-vinyl-2-(2′-methylethenyl)benzene was used in place of 1,2-divinylbenzene, NHC rotation was observed to occur, but not exchange between η2-isomers. These experiments provide insight into dynamic solution behaviour that is difficult to probe using static methods such as X-ray crystallography. A third study evaluated the behaviour of complexes derived from the reaction of 39 with 1,2-divinylbenzene; DFT calculations agreed with the observed solution behaviour with regards to the proportions of each conformer that were observed.32
Plenio explored the postulated ‘release-return mechanism’14,15 in reactions catalysed by pre-catalysts such as GH2.119 Hoveyda and co-workers had successfully isolated a proportion of GH1 from metathesis reactions, and therefore reasoned that the chelating iso-propoxystyrene ligand must return to the metal centre after reaction. However, Plenio and co-workers studied reactions catalysed by fluorescence-labelled 40 and fluorine-tagged 41, and did not observe the return of the ether ligand. The authors proposed that the recovered complex was uninitiated pre-catalyst; concentration/time data for the RCM of diethyl diallylmalonate reported by Percy et al. was later reported where complete conversion was obtained when only a fraction of the pre-catalyst had undergone initiation.115 Recent work by Solans-Monfort and co-workers supports this view.120
E/Z selectivity is key in metathesis: one isomer is typically desired in synthetic applications, while in polymer chemistry selectivity is necessary for a regular repeating structure. E/Z selectivity in CM reactions can be linked to several factors. As discussed above, second-generation pre-catalysts tend to lead to higher E-selectivities in CM reactions,76 due to their ability to equilibrate E/Z mixtures of products to the thermodynamically-favourable E-isomer. The structure of the ligands of second generation pre-catalysts can affect the E/Z ratio;121,122 for NHC–phosphine complexes, this ratio is typically ca. 3–10 (or higher), while for bis(NHC) complexes it can be ca. 2.
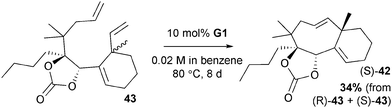
In RCM reactions, the thermodynamic product (E or Z) will depend on the structure of the product. For common and medium rings (5–10 members) the cis-isomer is typically preferred, due to the vast difference in strain energy between the cis- and trans-isomers.101 There are few examples of trans-configured eight-membered rings. Prunet and co-workers reported the preparation of trans-42via RCM of substrate (S)-43 (Scheme 20);77 corresponding (R)-43 was unreactive. This appears to be an isolated example of where dense functionalisation renders the trans-isomer more favourable than the cis-isomer; the fused cycloalkene and carbonate, plus the heavy substitution of most positions around the ring, clearly influences the energetics of the ring-closing reaction.
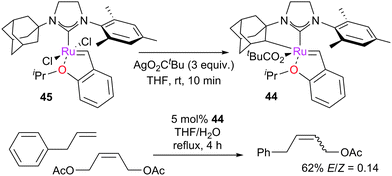
A key development in metathesis has been the design of Z-selective catalysts.123,124 Hoveyda and Schrock reported molybdenum- and tungsten-based Z-selective catalysts in 2009,125,126 while Grubbs reported the first ruthenium-based Z-selective catalyst 44 in 2011 (Scheme 21).12745, bearing an unsymmetrical 1-adamantyl-3-mesityl-substituted NHC, underwent reaction with AgO2CtBu to yield cyclometallated complex 44. When tested in the CM reaction of allylbenzene with acetyl-protected but-2-ene-1,4-diol, 44 led to E/Z ratios as low as 0.14. However, 44 was shown to be far slower for the RCM of diethyl diallylmalonate than GH2, even at higher temperatures and with much higher catalyst loadings.
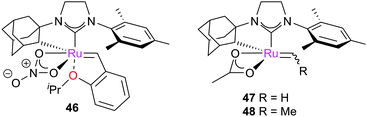
In a subsequent study, the effect of the pre-catalyst structure on reactivity was examined.128 Complexes such as 46, bearing chelating unsymmetrical NHCs and bidentate κ2-nitrato ligands were found to be the most active, achieving modest to excellent yields and E/Z ratios of typically 0.25 or lower. Studies suggested that a bulkier ligand sphere increased the initiation rate of the catalyst. This result, combined with the octahedral coordination sphere of the metal centre, suggests that an interchange or associative mechanism is unlikely, and that a dissociative mechanism is in operation for initiation.
Computational studies have been carried out in order to investigate why the chelate nature of the NHC and the replacement of the halide ligands stimulates a switch in selectivity from E to Z. Houk et al. investigated the reactivity of model 16e− species 47 and 48 with ethene and propene.129 Calculations of the degenerate exchange of ethene with 47 revealed that the chelated complexes strongly preferred the side-bound reaction, in contrast to G2.130 This is due to the chelating adamantyl substituent, which renders complexes where the alkylidene is trans- to the Ru–C σ-bond highly unfavourable, and introduces steric repulsion in the TSs for bottom-bound (retro)metallacyclobutanation. Side-bound TSs suffer far less steric repulsion, and d–π*NHC and d–π*alkylidene back-donation can occur via different, perpendicular d-orbitals. Side-bound MCBs were found to be far more stable (versus47, ΔG = −7.3 (side-bound), + 9.4 (bottom-bound) kcal mol−1). Consideration of the metathesis of propene with 48 revealed that side-bound MCBs were favoured; in these intermediates, TSs leading to the Z-olefin (ΔG‡ = 14.4 and 14.6 kcal mol−1) were more favourable, as these allowed both methyl groups to point downwards, away from the bulky NHC, while the E-selective TSs (ΔG‡ = 16.1 and 18.8 kcal mol−1) require at least one methyl group to be orientated towards the NHC.
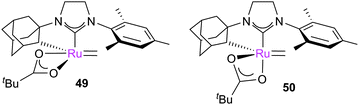
Additional computational studies regarding this selectivity have been conducted by Wang et al., who considered more steps of the mechanism.131 The initiation event was modelled according to the originally proposed dissociative mechanism,26,40,132 due to the coordinatively-saturated nature of the ruthenium centre (vide supra); interchange and associative pathways37–39 were not considered. Dissociation was followed by facile isomerisation of the square-based pyramidal complex 49 to trigonal bipyramidal complex 50, which was considered as the active species. The various pathways via which the reaction can proceed were modelled in detail; while the E-isomer was favoured thermodynamically, the kinetics favour Z-isomer formation. The authors confirmed that, while there were several other TSs on the PES, the key TS involves retro-[2+2]-cycloaddition to break up the MCB intermediate, where steric interactions with the NHC ligand are key. A later study confirmed that nitrato-analogues of these catalysts function in a similar way,133 with the ability of the ligand to switch between mono- and bi-dentate coordination modes key to the activity.
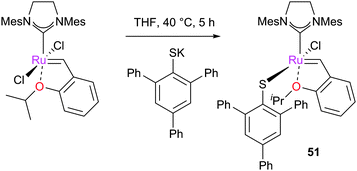
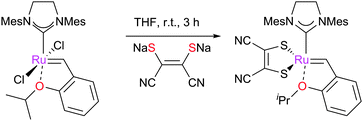
More recently, Jensen and co-workers disclosed the synthesis and study of an accessible Z-selective metathesis pre-catalyst. Simple exchange of a chloride ligand on GH2 with an arylthiol potassium salt led to complex 51, which showed excellent Z-selectivity (Scheme 22).134 Similarly, Hoveyda and co-workers have developed a different approach using the 2,3-dimercaptomaleonitrile ligand which, due to the forced cis-anion conformation, promotes the formation of Z-olefins in high selectivity and yields (Scheme 23).136
A DFT study on the stereochemical processes occurring around various types of (a)chiral ruthenium carbene complexes has recently carried out by Hoveyda et al.135,136 Their calculations indicated that the typical preference for bottom-bound MCBs was a consequence of destabilising the side-bound alternative (which will have cis-anionic ligands) via electron–electron repulsion and a large dipole moment. Ligand spheres which destabilise the usual square-based pyramidal geometry of metathesis catalysts can favour side-bound MCBs and therefore Z-selective metathesis. In chelated NHC complexes such as 46, the other anionic ligand is proposed to prefer to be trans to the NHC rather than the alkyl ligand, favouring a side-bound MCB.
Catalyst decomposition
Designing and utilising ligand environments that reduce deactivation pathways is always one of the major challenges in organometallic chemistry. However, to develop new and more efficient catalysts, understanding the decomposition processes is fundamentally important. Chemists seek to understand why side products may be formed during complex synthesis and during metathesis reactions, and also to evaluate the compatibility of catalysts with different chemical environments (e.g. to understand catalyst deactivation). In addition several catalyst decomposition products can react with metathesis substrates; therefore, it is very important to understand what these complexes are and which reactions they can catalyse.Methylidene complexes
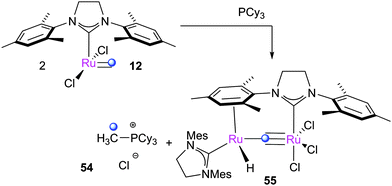
Methylidene complexes, formed after catalytic turnover with terminal alkenes, are often viewed as the most fragile species in metathesis reactions. During propagation, species 11 and 12 can re-coordinate the phosphine ligand, forming the corresponding 16e− methylidene species 52 and 53 (Scheme 24).Even though 52 and 53 are not formally decomposition products, they are prone to rapid decomposition, to the extent that their initiation rates cannot be measured.25 Their instability was proposed by Grubbs et al. to be due to rapid decomposition during which the phosphine dissociates and reacts with the alkylidene moiety, forming the phosphine ylide species 54 and dinuclear ruthenium species 55 (Scheme 25).137,138
Although the inorganic products of the decomposition of first generation 52 are still unknown, a bimetallic hydride species was isolated from decomposition of G2-derived 53. The proposed mechanism for this reaction goes via dissociation of a phosphine, which reacts with the alkylidene, forming phosphonium ylide Cy3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2. The postulated 12e− ruthenium product can then co-ordinate the mesityl ring of another molecule of 12, eventually leading to hydride complex 55 after HCl removal by Cy3P
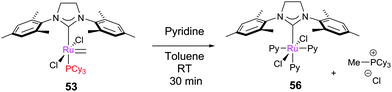
CH2. The postulated 12e− ruthenium product can then co-ordinate the mesityl ring of another molecule of 12, eventually leading to hydride complex 55 after HCl removal by Cy3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 liberated previously. 55 was shown to be active for the isomerisation of allylbenzene, but Fogg et al. later cast doubt on whether the activity of this species can account for all of the observed isomerisation in metathesis reactions.139 Different behaviour occurs in the presence of ethene (1 atm.), where 12 dimerises to form a chloride bridged cyclometallated species. Grubbs et al. screened the stability of a range of methylidene complexes, recording decomposition rates that were highly dependent on the ligand structure (Table 5). Methylidene complexes are also known to be sensitive to pyridine; for example, 53 reacts rapidly to form tris(pyridine) complex 56 (Scheme 26).138
CH2 liberated previously. 55 was shown to be active for the isomerisation of allylbenzene, but Fogg et al. later cast doubt on whether the activity of this species can account for all of the observed isomerisation in metathesis reactions.139 Different behaviour occurs in the presence of ethene (1 atm.), where 12 dimerises to form a chloride bridged cyclometallated species. Grubbs et al. screened the stability of a range of methylidene complexes, recording decomposition rates that were highly dependent on the ligand structure (Table 5). Methylidene complexes are also known to be sensitive to pyridine; for example, 53 reacts rapidly to form tris(pyridine) complex 56 (Scheme 26).138
Deactivation by substrates
Methylidene complexes are not the only vectors for decomposition: reactions with certain substrates can lead to unwanted side reactions of the catalyst.Cyclopropenyl substrates
When substrates such as Feist ester 57 are employed, G1 and G2 decompose to ruthenium carbide complexes 58 and 59, respectively. The proposed mechanism involves the formation of species 60 or 61 which rearranges, eliminating dimethyl fumarate and the carbide complex 58 and 59 (Scheme 27).140 Notably, protonation of these species with [H(OEt2)2][B(C6F5)] leads to Piers-type rapidly-initiating catalysts.17 [RuCl2(PPh3)2(CHPh)] reacts with the ester to yield only the intermediate carbene.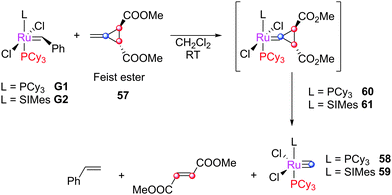 | ||
| Scheme 27 Decomposition of alkene metathesis pre-catalysts via reaction with cyclopropenyl substrates. | ||
Electron-rich alkenes
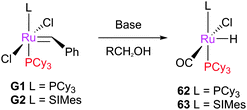
Reactions with vinyl halides have been reported to lead to decomposition in some cases, driven by the stability of the product Fischer-type carbenes.141 Indeed, these are often used as a catalyst quench or for the determination of initiation rate.Grubbs et al. prepared a series of Fischer carbene complexes via the metathesis of vinyl ethers (Scheme 28).71 The product complexes were found to be much less active than the parent benzylidene species, requiring high temperatures to achieve turnover. In addition, thermolysis of these species leads to ruthenium hydride species (such as 62 and 63) which may isomerise substrate and product alkenes.139,142–146
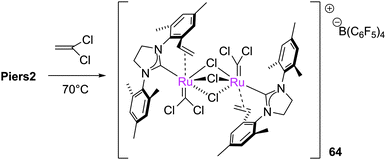
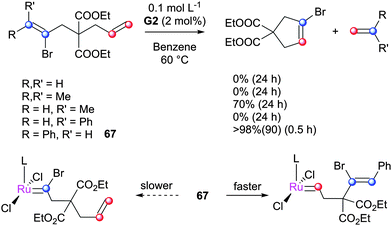
The reaction of Piers2 with 1,1-dichloroethene leads to the halide bridged complex 64 (Scheme 29).147 In addition, catalytically-inactive species 58, 59, 65 and 66 have been obtained from the reaction of vinyl halides and vinyl esters with metathesis pre-catalysts (Scheme 30).148–150 In some cases the metathesis of vinyl halides can be successful. Stoltz reported the synthesis of elatol, via a spirocyclic intermediate bearing a vinyl chloride.72,151 Notably, the methodology used avoids the formation of an α-chloro alkylidene intermediate. Dorta and co-workers conducted a rational study to elucidate how RCM to form such halogenated alkenes might be achieved, by analysing the results of the RCM of a series of vinyl bromides (Scheme 31).152 However, only diene 67, with a phenyl substituent cis to the bromide, achieved complete conversion to the product. In the Dorta example, formation of an α-haloalkylidene might also be avoided, if reaction with the alternative terminus is faster.
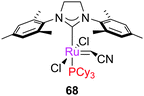
Acrylonitrile is traditionally a very difficult substrate, due to the formation of inactive complexes such as 68. The use of phosphine-free catalysts such as G2-py can overcome this issue, by precluding the capture of 14e− alkylidenes by phosphine.13
Ligand C–H activation
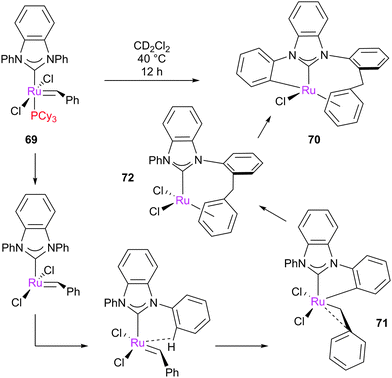
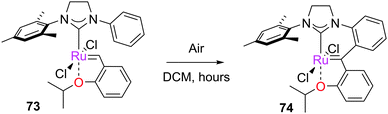
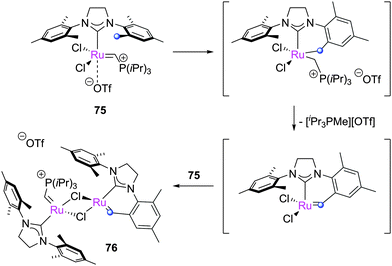
When the N-aryl substituents on the imidazolium ring can rotate to interact with the ruthenium centre, C–H activation can occur. There are two possible types of C–H insertion: to form ruthenium metallacycles, or insertion of the alkylidene moiety. Grubbs reported the spontaneous double C–H activation of 69 to form 70 (Scheme 32).153 This interesting rearrangement has been computationally studied by Cavallo and Suresh;154–156 the N-phenyl substituent, due to the relatively low barriers to rotation, can be ortho-metallated, obtaining intermediate 71, which immediately rearranges to complex 72. This complex can insert into the C–H on the other N-phenyl moiety, achieving the final product 70. Bulkier N-aryl substituents impede this rotation, precluding the C–H activation process.A different C–H insertion process can occur via the alkylidene moiety. This decomposition can occur, for example, in the presence of oxygen. Blechert proposed that 73 can undergo intramolecular C–H insertion into the alkylidene moiety through a pericyclic rearrangement of the aryl substituent to form the carbene arene complex 74 (Scheme 33).157 C–H insertion can also occur if the catalyst is not thermally stable in solution; for example, Piers reported that pre-catalyst 75, after two days at room temperature, dimerised to form complex 76 (Scheme 34).
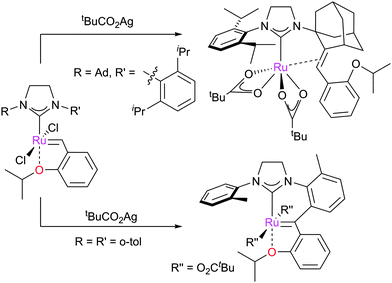
In certain cases, cyclometallation of the ligand allows the generation of new pre-catalysts with new reactivity. For example, 45 underwent reaction with tBuCO2Ag, followed by insertion of the ruthenium centre into a C–H bond on the N-adamantyl substituent to form complex 44 (Scheme 21, above).128,158 This complex was revealed to be Z-selective in CM reactions. This methodology has been studied in detail with several pre-catalyst motifs, revealing that the synthetic route to 44 is specific to this NHC (Scheme 35). Increasing the bulkiness of the N-aryl substituent or changing the adamantyl substituent to an aromatic moiety caused decomposition.159
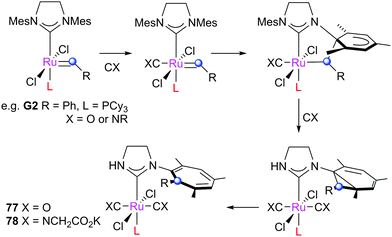
π-Acids
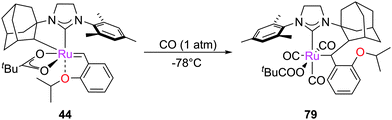
π-Acids such as CO react with pre-catalysts, forming metathesis-inactive decomposition products. First reported by Diver, exposing G2 or methylidene 53 to 1 atm. CO at room temperature results in the rapid rearrangement of the carbene moiety, which inserts into the N-aryl substituent via a Buchner-type mechanism, leading to complexes such as 77 (Scheme 36).160 Similar reactivity was demonstrated with isocyanides and with different second generation pre-catalysts.161 An interesting application is the use of CNCH2CO2K as a catalyst scavenger, which generates complexes such as 78 that can be easily removed from the reaction mixture.162 The mechanism of this reaction has been investigated computationally by Cavallo, who suggested that it proceeds via reaction of the ipso carbon of the N-aryl substituent with the carbene, promoted by the π-acidity of the ligand trans to the alkylidene.163Another decomposition route involving CO was reported by Grubbs with cyclometallated complex 44. It was found that at −78 °C in the presence of excess CO, the co-ordination of CO to the ruthenium centre promotes the C–H insertion of the N-adamantyl substituent into the carbene moiety, achieving complex 79 (Scheme 37).159 This C–H activation is due to the ligand substitution by the π-acidic CO ligands, which decreases the ability of the metal centre to stabilise the alkylidene by back-bonding. This lack of stabilisation is presumably relieved by the C–H N-adamantyl substituent in the alkylidene forming inactive complex 79.
Alcoholysis
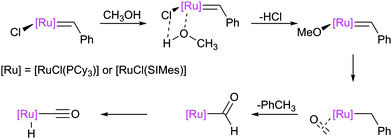
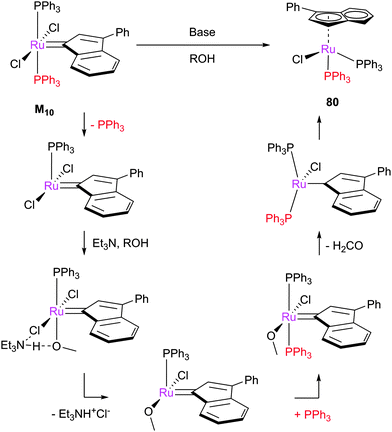
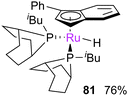
The alcoholysis of metathesis pre-catalysts in the presence of base has been reported to lead to (typically hydride) decomposition products, which are interesting not only as decomposition products but as catalysts in tandem metathesis/hydrogenation or metathesis/isomerisation processes.164,165 Mol et al. carried out a number of studies, stirring complexes such as G1 and G2 in methanol in the presence of a base. This caused decomposition to hydridocarbonyl complexes 62 and 63 respectively (Scheme 38).144–146 A mechanism for the alcoholysis reaction was tentatively suggested by Mol, which was somewhat supported by labelling experiments. Notably, the use of alcohols such as ethanol in this process means that a carbon–carbon bond breaking process must occur during the reaction; 13C labelling experiments confirmed that the ethanol was the source of the CO ligand. This mechanism was recently studied by Percy, Hillier and Tuttle using DFT calculations (Scheme 39).142,145Different behaviour was observed with indenylidene species M10 and M11, while M1 behaved as G1. In basic alcohol solution, M10 (a starting material for most indenylidene pre-catalysts) undergoes reaction to form an η5-indenyl species [RuCl(η5-3-phenylindenyl)(PPh3)2] 80 (Scheme 40).166 This new complex has been found to be highly active in a number of transformations, from alcohol racemisation to carboxylic acid reduction.167 Analogous complex M11 undergoes a similar reaction, but reacts further to form hydride species 81.168
A subsequent detailed study established that PCy3-bearing complexes such as G1, M1, and G1-derived 52 decomposed in primary alcohols to yield hydridocarbonyl complex 62, and in secondary alcohols to yield hydrogenohydride species 82 (Scheme 41).169 Notably, the synthesis of the latter species requires the use of dihydrogen in all other reports. The different behaviour of complexes bearing different phosphine ligands was rationalised by DFT studies of the potential energy surfaces for formation of the η5-complexes bearing triphenylphosphine, tricyclohexylphosphine, and iso-butylphoban ligands.
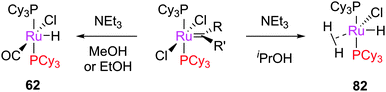 | ||
| Scheme 41 Decomposition of tricyclohexylphosphine-containing complexes in primary versus secondary alcohols. | ||
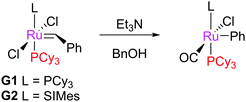
Treating G1 and G2 with benzyl alcohol, and a base such as triethylamine, does not form the expected hydridocarbonyl complexes, but a different species where the phenyl is directly bonded to the ruthenium centre (Scheme 42).145 The SIMes-bearing product was also obtained when G2 was exposed to oxygen, although other side products, such as 83, have also been attributed to the reaction of G2 with oxygen.170
Other routes
Van Rensburg and co-workers have studied the decomposition of ruthenium metathesis catalysts via hydride abstraction in the MCB to yield an η3-allyl complex (Scheme 43).171,172 The observed decomposition products (experimentally) were in agreement with the calculated decomposition route.142Conclusions and outlook
Recent research in the field of alkene metathesis, most of it within the last 15 years or so, has improved our understanding of mechanistic details of alkene metathesis reactions. The initiation of a variety of pre-catalysts, key steps during the catalytic cycle, and the decomposition of metathesis (pre-)catalysts have all been explored by a range of researchers, using a number of techniques such as NMR spectroscopy, DFT studies, and mass spectrometry. Highlights include: a detailed understanding of pre-catalyst initiation, including how various structural features influence the rate of this process and therefore the rate of delivery of the active catalyst into solution; probing of key steps of the mechanism such as the formation and breakdown of MCBs and how these depend on catalyst and substrate structure; and the characterisation and redeployment of interesting decomposition products. These studies have helped to guide the development of future catalysts and reactions. It is now well-accepted that there is no single ‘best’ pre-catalyst; mechanistic details available in the literature allow the end users of metathesis technology to select an appropriate balance of initiation rate and thermal stability, based on their specific application. Future work in the area will undoubtedly continue to yield new catalysts with new reactivity profiles, as well as further elegant uses of metathesis techniques in synthetic and materials chemistry.Acknowledgements
Work on metathesis chemistry within our research group has received funding from a number of sources, to whom we are very grateful. These include: the EPSRC, the ERC (via Advanced Investigator Grant ‘FUNCAT’ to SPN), the EC (via FP7 project ‘EUMET’) and Umicore (for gifts of materials). SPN is a Royal Society Wolfson Research Merit Award holder.Notes and references
- Y. Chauvin, Angew. Chem., Int. Ed., 2006, 45, 3740–3747 CrossRef PubMed
.
- R. H. Grubbs, Angew. Chem., Int. Ed., 2006, 45, 3760–3765 CrossRef CAS PubMed
.
- R. R. Schrock, Angew. Chem., Int. Ed., 2006, 45, 3748–3759 CrossRef CAS PubMed
.
- D. Astruc, New J. Chem., 2005, 29, 42–56 RSC
.
-
G. C. Lloyd-Jones, in The Investigation of Organic Reactions and Their Mechanisms, ed. H. Maskill, Wiley Blackwell, Oxford, 2006, pp. 343–352 Search PubMed
.
- C. Samojlowicz, M. Bieniek and K. Grela, Chem. Rev., 2009, 109, 3708–3742 CrossRef CAS PubMed
.
- G. C. Vougioukalakis and R. H. Grubbs, Chem. Rev., 2009, 110, 1746–1787 CrossRef PubMed
.
- S. Díez-González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef PubMed
.
- P. J.-L. Hérisson and Y. Chauvin, Makromol. Chem., 1970, 141, 161–176 CrossRef
.
- F. Boeda, H. Clavier and S. P. Nolan, Chem. Commun., 2008, 2726–2740 RSC
.
- P. Schwab, M. B. France, J. W. Ziller and R. H. Grubbs, Angew. Chem., Int. Ed. Engl., 1995, 34, 2039–2041 CrossRef CAS
.
- M. Scholl, S. Ding, C. W. Lee and R. H. Grubbs, Org. Lett., 1999, 1, 953–956 CrossRef CAS
.
- J. A. Love, J. P. Morgan, T. M. Trnka and R. H. Grubbs, Angew. Chem., Int. Ed., 2002, 41, 4035–4037 CrossRef CAS
.
- S. B. Garber, J. S. Kingsbury, B. L. Gray and A. H. Hoveyda, J. Am. Chem. Soc., 2000, 122, 8168–8179 CrossRef CAS
.
- J. S. Kingsbury, J. P. A. Harrity, P. J. Bonitatebus and A. H. Hoveyda, J. Am. Chem. Soc., 1999, 121, 791–799 CrossRef CAS
.
- A. Michrowska, R. Bujok, S. Harutyunyan, V. Sashuk, G. Dolgonos and K. Grela, J. Am. Chem. Soc., 2004, 126, 9318–9325 CrossRef CAS PubMed
.
- P. E. Romero, W. E. Piers and R. McDonald, Angew. Chem., Int. Ed., 2004, 43, 6161–6165 CrossRef CAS PubMed
.
- S. R. Dubberley, P. E. Romero, W. E. Piers, R. McDonald and M. Parvez, Inorg. Chim. Acta, 2006, 359, 2658–2664 CrossRef CAS PubMed
.
- J. Huang, E. D. Stevens, S. P. Nolan and J. L. Petersen, J. Am. Chem. Soc., 1999, 121, 2674–2678 CrossRef CAS
.
- R. Credendino, A. Poater, F. Ragone and L. Cavallo, Catal. Sci. Technol., 2011, 1, 1287–1297 CAS
.
- J. I. du Toit, C. G. C. E. van Sittert and H. C. M. Vosloo, J. Organomet. Chem., 2013, 738, 76–91 CrossRef CAS PubMed
.
- E. L. Dias, S. T. Nguyen and R. H. Grubbs, J. Am. Chem. Soc., 1997, 119, 3887–3897 CrossRef CAS
.
- M. S. Sanford, M. Ulman and R. H. Grubbs, J. Am. Chem. Soc., 2001, 123, 749–750 CrossRef CAS
.
- D. J. Nelson and S. P. Nolan, Chem. Soc. Rev., 2013, 42, 6723–6753 RSC
.
- M. S. Sanford, J. A. Love and R. H. Grubbs, J. Am. Chem. Soc., 2001, 123, 6543–6554 CrossRef CAS PubMed
.
- J. A. Love, M. S. Sanford, M. W. Day and R. H. Grubbs, J. Am. Chem. Soc., 2003, 125, 10103–10109 CrossRef CAS PubMed
.
- I. W. Ashworth, D. J. Nelson and J. M. Percy, Dalton Trans., 2013, 42, 4110–4113 RSC
.
- C. Samojłowicz, M. Bieniek, A. Pazio, A. Makal, K. Woźniak, A. Poater, L. Cavallo, J. Wójcik, K. Zdanowski and K. Grela, Chem. – Eur. J., 2011, 17, 12981–12993 CrossRef PubMed
.
- C. Samojlowicz, M. Bieniek, A. Zarecki, R. Kadyrov and K. Grela, Chem. Commun., 2008, 6282–6284 RSC
.
- K. Getty, M. U. Delgado-Jaime and P. Kennepohl, J. Am. Chem. Soc., 2007, 129, 15774–15776 CrossRef CAS PubMed
.
- Y. Zhao and D. G. Truhlar, Org. Lett., 2007, 9, 1967–1970 CrossRef CAS PubMed
.
- I. C. Stewart, D. Benitez, D. J. O'Leary, E. Tkatchouk, M. W. Day, W. A. Goddard and R. H. Grubbs, J. Am. Chem. Soc., 2009, 131, 1931–1938 CrossRef CAS PubMed
.
- Y. Minenkov, G. Occhipinti, W. Heyndrickx and V. R. Jensen, Eur. J. Inorg. Chem., 2012, 1507–1516 CrossRef CAS
.
- H.-C. Yang, Y.-C. Huang, Y.-K. Lan, T.-Y. Luh, Y. Zhao and D. G. Truhlar, Organometallics, 2011, 30, 4196–4200 CrossRef CAS
.
- C. A. Urbina-Blanco, A. Poater, T. Lebl, S. Manzini, A. M. Z. Slawin, L. Cavallo and S. P. Nolan, J. Am. Chem. Soc., 2013, 135, 7073–7079 CrossRef CAS PubMed
.
- G. C. Vougioukalakis and R. H. Grubbs, Chem. – Eur. J., 2008, 14, 7545–7556 CrossRef CAS PubMed
.
- T. Vorfalt, K. J. Wannowius and H. Plenio, Angew. Chem., Int. Ed., 2010, 49, 5533–5536 CrossRef CAS PubMed
.
- I. W. Ashworth, I. H. Hillier, D. J. Nelson, J. M. Percy and M. A. Vincent, Chem. Commun., 2011, 47, 5428–5430 RSC
.
- V. Thiel, M. Hendann, K.-J. Wannowius and H. Plenio, J. Am. Chem. Soc., 2011, 134, 1104–1114 CrossRef PubMed
.
- F. Nuñez-Zarur, X. Solans-Monfort, L. Rodríguez-Santiago and M. Sodupe, Organometallics, 2012, 31, 4203–4215 CrossRef
.
- D. J. Nelson and J. M. Percy, Dalton Trans., 2014, 43, 4674–4679 RSC
.
- D. J. Nelson, P. Queval, M. Rouen, M. Magrez, L. Toupet, F. Caijo, E. Borré, I. Laurent, C. Crévisy, O. Baslé, M. Mauduit and J. M. Percy, ACS Catal., 2013, 3, 259–264 CrossRef CAS
.
- I. W. Ashworth, I. H. Hillier, D. J. Nelson, J. M. Percy and M. A. Vincent, ACS Catal., 2013, 3, 1929–1939 CrossRef CAS
.
- M. Barbasiewicz, A. Szadkowska, A. Makal, K. Jarzembska, K. Woźniak and K. Grela, Chem. – Eur. J., 2008, 14, 9330–9337 CrossRef CAS PubMed
.
- M. Barbasiewicz, K. Grudzień and M. Malinska, Organometallics, 2012, 31, 3171–3177 CrossRef CAS
.
- P. E. Romero and W. E. Piers, J. Am. Chem. Soc., 2005, 127, 5032–5033 CrossRef CAS PubMed
.
- P. E. Romero and W. E. Piers, J. Am. Chem. Soc., 2007, 129, 1698–1704 CrossRef CAS PubMed
.
- E. F. van der Eide, P. E. Romero and W. E. Piers, J. Am. Chem. Soc., 2008, 130, 4485–4491 CrossRef CAS PubMed
.
- E. M. Leitao, E. F. v. d. Eide, P. E. Romero, W. E. Piers and R. McDonald, J. Am. Chem. Soc., 2010, 132, 2784–2794 CrossRef CAS PubMed
.
- E. F. van der Eide and W. E. Piers, Nat. Chem., 2010, 2, 571–576 CrossRef CAS PubMed
.
- A. G. Wenzel and R. H. Grubbs, J. Am. Chem. Soc., 2006, 128, 16048–16049 CrossRef CAS PubMed
.
- A. G. Wenzel, G. Blake, D. G. VanderVelde and R. H. Grubbs, J. Am. Chem. Soc., 2011, 133, 6429–6439 CrossRef CAS PubMed
.
- B. K. Keitz and R. H. Grubbs, J. Am. Chem. Soc., 2011, 133, 16277–16284 CrossRef CAS PubMed
.
- A. K. Chatterjee, T.-L. Choi, D. P. Sanders and R. H. Grubbs, J. Am. Chem. Soc., 2003, 125, 11360–11370 CrossRef CAS PubMed
.
- C. A. Tolman, Chem. Rev., 1977, 77, 313–348 CrossRef CAS
.
- X. Bantreil, T. E. Schmid, R. A. M. Randall, A. M. Z. Slawin and C. S. J. Cazin, Chem. Commun., 2010, 46, 7115–7117 RSC
.
- X. Bantreil, A. Poater, C. A. Urbina-Blanco, Y. D. Bidal, L. Falivene, R. A. M. Randall, L. Cavallo, A. M. Z. Slawin and C. S. J. Cazin, Organometallics, 2012, 31, 7415–7426 CrossRef CAS
.
- T. E. Schmid, X. Bantreil, C. A. Citadelle, A. M. Z. Slawin and C. S. J. Cazin, Chem. Commun., 2011, 47, 7060–7062 RSC
.
- C. A. Urbina-Blanco, X. Bantreil, J. Wappel, T. E. Schmid, A. M. Z. Slawin, C. Slugovc and C. S. J. Cazin, Organometallics, 2013, 32, 6240–6247 CrossRef CAS
.
- S. Monsaert, A. Lozano Vila, R. Drozdzak, P. Van Der Voort and F. Verpoort, Chem. Soc. Rev., 2009, 38, 3360–3372 RSC
.
- Y. Vidavsky, A. Anaby and N. G. Lemcoff, Dalton Trans., 2012, 41, 32–43 RSC
.
- M. Barbasiewicz, A. Szadkowska, R. Bujok and K. Grela, Organometallics, 2006, 25, 3599–3604 CrossRef CAS
.
- A. Poater, F. Ragone, A. Correa, A. Szadkowska, M. Barbasiewicz, K. Grela and L. Cavallo, Chem. – Eur. J., 2010, 16, 14354–14364 CrossRef CAS PubMed
.
- E. Tzur, A. Szadkowska, A. Ben-Asuly, A. Makal, I. Goldberg, K. Woźniak, K. Grela and N. G. Lemcoff, Chem. – Eur. J., 2010, 16, 8726–8737 CrossRef CAS PubMed
.
- M. Barbasiewicz, M. Michalak and K. Grela, Chem. – Eur. J., 2012, 18, 14237–14241 CrossRef CAS PubMed
.
- K. Grudzień, K. Żukowska, M. Malińska, K. Woźniak and M. Barbasiewicz, Chem. – Eur. J., 2014, 20, 2819–2828 CrossRef PubMed
.
- K. Żukowska, A. Szadkowska, B. Trzaskowski, A. Pazio, Ł. Pączek, K. Woźniak and K. Grela, Organometallics, 2013, 32, 2192–2198 CrossRef
.
- B. Trzaskowski and K. Grela, Organometallics, 2013, 32, 3625–3630 CrossRef CAS
.
- T. Weskamp, W. C. Schattenmann, M. Spiegler and W. A. Herrmann, Angew. Chem., Int. Ed., 1998, 37, 2490–2493 CrossRef CAS
.
- M. Scholl, T. M. Trnka, J. P. Morgan and R. H. Grubbs, Tetrahedron Lett., 1999, 40, 2247–2250 CrossRef CAS
.
- J. Louie and R. H. Grubbs, Organometallics, 2002, 21, 2153–2164 CrossRef CAS
.
- D. E. White, I. C. Stewart, R. H. Grubbs and B. M. Stoltz, J. Am. Chem. Soc., 2007, 130, 810–811 CrossRef PubMed
.
- M. Bieniek, A. Michrowska, D. L. Usanov and K. Grela, Chem. – Eur. J., 2008, 14, 806–818 CrossRef CAS PubMed
.
- S. Monfette and D. E. Fogg, Chem. Rev., 2009, 109, 3783–3816 CrossRef CAS PubMed
.
- D. J. Nelson, I. W. Ashworth, I. H. Hillier, S. H. Kyne, S. Pandian, J. A. Parkinson, J. M. Percy, G. Rinaudo and M. A. Vincent, Chem. – Eur. J., 2011, 17, 13087–13094 CrossRef CAS PubMed
.
- T. Ritter, A. Hejl, A. G. Wenzel, T. W. Funk and R. H. Grubbs, Organometallics, 2006, 25, 5740–5745 CrossRef CAS
.
- D. Bourgeois, A. Pancrazi, L. Ricard and J. Prunet, Angew. Chem., Int. Ed., 2000, 39, 725–728 CrossRef CAS
.
- A. Gradillas and J. P. Castells, Angew. Chem., Int. Ed., 2006, 45, 6086–6101 CrossRef CAS PubMed
.
- L. Cavallo, J. Am. Chem. Soc., 2002, 124, 8965–8973 CrossRef CAS PubMed
.
- B. F. Straub, Angew. Chem., Int. Ed., 2005, 44, 5974–5978 CrossRef CAS PubMed
.
- B. F. Straub, Adv. Synth. Catal., 2007, 349, 204–214 CrossRef CAS
.
- I. Fernández, N. Lugan and G. Lavigne, Organometallics, 2012, 31, 1155–1160 CrossRef
.
- M. Sussner and H. Plenio, Chem. Commun., 2005, 5417–5419 RSC
.
- S. Leuthäußer, V. Schmidts, C. M. Thiele and H. Plenio, Chem. – Eur. J., 2008, 14, 5465–5481 CrossRef PubMed
.
- R. Credendino, L. Falivene and L. Cavallo, J. Am. Chem. Soc., 2012, 134, 8127–8135 CrossRef CAS PubMed
.
- J.-H. Sohn, K. H. Kim, H.-Y. Lee, Z. S. No and H. Ihee, J. Am. Chem. Soc., 2008, 130, 16506–16507 CrossRef CAS
.
- K. H. Kim, T. Ok, K. Lee, H.-S. Lee, K. T. Chang, H. Ihee and J.-H. Sohn, J. Am. Chem. Soc., 2010, 132, 12027–12033 CrossRef CAS PubMed
.
- L. Benhamou, E. Chardon, G. Lavigne, S. Bellemin-Laponnaz and V. César, Chem. Rev., 2011, 111, 2705–2733 CrossRef CAS PubMed
.
- H. Clavier and S. P. Nolan, Chem. Commun., 2010, 46, 841–861 RSC
.
- F. Ragone, A. Poater and L. Cavallo, J. Am. Chem. Soc., 2010, 132, 4249–4258 CrossRef CAS PubMed
.
- A. Poater, B. Cosenza, A. Correa, S. Giudice, F. Ragone, V. Scarano and L. Cavallo, Eur. J. Inorg. Chem., 2009, 1759–1766 CrossRef CAS
.
- T. Dröge and F. Glorius, Angew. Chem., Int. Ed., 2010, 49, 6940–6952 CrossRef PubMed
.
- R. M. Thomas, B. K. Keitz, T. M. Champagne and R. H. Grubbs, J. Am. Chem. Soc., 2011, 133, 7490–7496 CrossRef CAS PubMed
.
- S. Kavitake, M. K. Samantaray, R. Dehn, S. Deuerlein, M. Limbach, J. A. Schachner, E. Jeanneau, C. Coperet and C. Thieuleux, Dalton Trans., 2011, 40, 12443–12446 RSC
.
- A. J. Kirby, Adv. Phys. Org. Chem., 1980, 17, 183–278 CrossRef CAS
.
- L. Mandolini, Adv. Phys. Org. Chem., 1986, 22, 1–111 CrossRef CAS
.
- J. C. Conrad, M. D. Eelman, J. A. D. Silva, S. Monfette, H. H. Parnas, J. L. Snelgrove and D. E. Fogg, J. Am. Chem. Soc., 2007, 129, 1024–1025 CrossRef CAS PubMed
.
- C. Galli and L. Mandolini, Eur. J. Org. Chem., 2000, 3117–3125 CrossRef CAS
.
- L. Mitchell, J. A. Parkinson, J. M. Percy and K. Singh, J. Org. Chem., 2008, 73, 2389–2395 CrossRef CAS PubMed
.
- I. W. Ashworth, D. Carboni, I. H. Hillier, D. J. Nelson, J. M. Percy, G. Rinaudo and M. A. Vincent, Chem. Commun., 2010, 46, 7145–7147 RSC
.
- H. A. Skinner and G. Pilcher, Q. Rev., Chem. Soc., 1963, 17, 264–288 RSC
.
- C. Hinderling, C. Adlhart and P. Chen, Angew. Chem., Int. Ed., 1998, 37, 2685–2689 CrossRef CAS
.
- C. Adlhart and P. Chen, Helv. Chim. Acta, 2000, 83, 2192–2196 CrossRef CAS
.
- C. Adlhart, C. Hinderling, H. Baumann and P. Chen, J. Am. Chem. Soc., 2000, 122, 8204–8214 CrossRef CAS
.
- C. Adlhart, M. A. O. Volland, P. Hofmann and P. Chen, Helv. Chim. Acta, 2000, 83, 3306–3311 CrossRef CAS
.
- S. Torker, D. Merki and P. Chen, J. Am. Chem. Soc., 2008, 130, 4808–4814 CrossRef CAS PubMed
.
- H. Wang and J. r. O. Metzger, Organometallics, 2008, 27, 2761–2766 CrossRef CAS
.
- H.-Y. Wang, W.-L. Yim, T. Kluner and J. O. Metzger, Chem. – Eur. J., 2009, 15, 10948–10959 CrossRef CAS PubMed
.
- H.-Y. Wang, W.-L. Yim, Y.-L. Guo and J. O. Metzger, Organometallics, 2012, 31, 1627–1634 CrossRef CAS
.
- C. E. Webster, J. Am. Chem. Soc., 2007, 129, 7490–7491 CrossRef CAS PubMed
.
- C. N. Rowley, E. F. van der Eide, W. E. Piers and T. K. Woo, Organometallics, 2008, 27, 6043–6045 CrossRef CAS
.
- I. C. Stewart, B. K. Keitz, K. M. Kuhn, R. M. Thomas and R. H. Grubbs, J. Am. Chem. Soc., 2010, 132, 8534–8535 CrossRef CAS PubMed
.
- M. Ulman and R. H. Grubbs, Organometallics, 1998, 17, 2484–2489 CrossRef CAS
.
- D. R. Lane, C. M. Beavers, M. M. Olmstead and N. E. Schore, Organometallics, 2009, 28, 6789–6797 CrossRef CAS
.
- D. J. Nelson, D. Carboni, I. W. Ashworth and J. M. Percy, J. Org. Chem., 2011, 76, 8386–8393 CrossRef CAS PubMed
.
- D. R. Anderson, D. D. Hickstein, D. J. O'Leary and R. H. Grubbs, J. Am. Chem. Soc., 2006, 128, 8386–8387 CrossRef CAS PubMed
.
- J. A. Tallarico, P. J. Bonitatebus and M. L. Snapper, J. Am. Chem. Soc., 1997, 119, 7157–7158 CrossRef CAS
.
- D. Anderson, D. O'Leary and R. Grubbs, Chem. – Eur. J., 2008, 14, 7536–7544 CrossRef CAS PubMed
.
- T. Vorfalt, K. J. Wannowius, V. Thiel and H. Plenio, Chem. – Eur. J., 2010, 16, 12312–12315 CrossRef CAS PubMed
.
- F. Núñez-Zarur, X. Solans-Monfort, R. Pleixats, L. Rodríguez-Santiago and M. Sodupe, Chem. – Eur. J., 2013, 19, 14553–14565 CrossRef PubMed
.
- C. Luján and S. P. Nolan, J. Organomet. Chem., 2011, 696, 3935–3938 CrossRef PubMed
.
- C. Luján and S. P. Nolan, Catal. Sci. Technol., 2012, 2, 1027–1032 Search PubMed
.
- S. Shahane, C. Bruneau and C. Fischmeister, ChemCatChem, 2013, 5, 3436–3459 CrossRef CAS
.
- A. Fürstner, Science, 2013, 341, 1229713 CrossRef PubMed
.
- M. M. Flook, A. J. Jiang, R. R. Schrock, P. Müller and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 7962–7963 CrossRef CAS PubMed
.
- I. Ibrahem, M. Yu, R. R. Schrock and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 3844–3845 CrossRef CAS PubMed
.
- K. Endo and R. H. Grubbs, J. Am. Chem. Soc., 2011, 133, 8525–8527 CrossRef CAS PubMed
.
- B. K. Keitz, K. Endo, P. R. Patel, M. B. Herbert and R. H. Grubbs, J. Am. Chem. Soc., 2011, 134, 693–699 CrossRef PubMed
.
- P. Liu, X. Xu, X. Dong, B. K. Keitz, M. B. Herbert, R. H. Grubbs and K. N. Houk, J. Am. Chem. Soc., 2012, 134, 1464–1467 CrossRef CAS PubMed
.
- D. Benitez, E. Tkatchouk and W. A. Goddard III, Chem. Commun., 2008, 6194–6196 RSC
.
- Y. Dang, Z.-X. Wang and X. Wang, Organometallics, 2012, 31, 7222–7234 CrossRef CAS
.
- X. Solans-Monfort, R. Pleixats and M. Sodupe, Chem. – Eur. J., 2010, 16, 7331–7343 CrossRef CAS PubMed
.
- Y. Dang, Z.-X. Wang and X. Wang, Organometallics, 2012, 31, 8654–8657 CrossRef CAS
.
- G. Occhipinti, F. R. Hansen, K. W. Törnroos and V. R. Jensen, J. Am. Chem. Soc., 2013, 135, 3331–3334 CrossRef CAS PubMed
.
- S. Torker, R. K. M. Khan and A. H. Hoveyda, J. Am. Chem. Soc., 2014, 136, 3439–3455 CrossRef CAS PubMed
.
- R. K. M. Khan, S. Torker and A. H. Hoveyda, J. Am. Chem. Soc., 2013, 135, 10258–10261 CrossRef CAS PubMed
.
- S. H. Hong, M. W. Day and R. H. Grubbs, J. Am. Chem. Soc., 2004, 126, 7414–7415 CrossRef CAS PubMed
.
- S. H. Hong, A. G. Wenzel, T. T. Salguero, M. W. Day and R. H. Grubbs, J. Am. Chem. Soc., 2007, 129, 7961–7968 CrossRef CAS PubMed
.
- C. S. Higman, L. Plais and D. E. Fogg, ChemCatChem, 2013, 5, 3548–3551 CrossRef CAS
.
- R. G. Carlson, M. A. Gile, J. A. Heppert, M. H. Mason, D. R. Powell, D. V. Velde and J. M. Vilain, J. Am. Chem. Soc., 2002, 124, 1580–1581 CrossRef CAS PubMed
.
- Y. Minenkov, G. Occhipinti and V. R. Jensen, Organometallics, 2013, 32, 2099–2111 CrossRef CAS
.
- I. W. Ashworth, I. H. Hillier, D. J. Nelson, J. M. Percy and M. A. Vincent, Eur. J. Org. Chem., 2012, 5673–5677 CrossRef CAS
.
- A. Young, M. A. Vincent, I. H. Hillier, J. M. Percy and T. Tuttle, Dalton Trans., 2014, 43, 8493–8498 RSC
.
- M. B. Dinger and J. C. Mol, Eur. J. Inorg. Chem., 2003, 2827–2833 CrossRef CAS
.
- M. B. Dinger and J. C. Mol, Organometallics, 2003, 22, 1089–1095 CrossRef CAS
.
- D. Banti and J. C. Mol, J. Organomet. Chem., 2004, 689, 3113–3116 CrossRef CAS PubMed
.
- E. Leitao, S. Dubberley, W. Piers, Q. Wu and R. McDonald, Chem. – Eur. J., 2008, 14, 11565–11572 CrossRef CAS PubMed
.
- M. L. Macnaughtan, M. J. A. Johnson and J. W. Kampf, J. Am. Chem. Soc., 2007, 129, 7708–7709 CrossRef CAS PubMed
.
- M. L. Macnaughtan, J. B. Gary, D. L. Gerlach, M. J. A. Johnson and J. W. Kampf, Organometallics, 2009, 28, 2880–2887 CrossRef CAS
.
- M. L. Macnaughtan, M. J. A. Johnson and J. W. Kampf, Organometallics, 2007, 26, 780–782 CrossRef CAS
.
- D. E. White, I. C. Stewart, B. A. Seashore-Ludlow, R. H. Grubbs and B. M. Stoltz, Tetrahedron, 2010, 66, 4668–4686 CrossRef CAS PubMed
.
- M. Gatti, E. Drinkel, L. Wu, I. Pusterla, F. Gaggia and R. Dorta, J. Am. Chem. Soc., 2010, 132, 15179–15181 CrossRef CAS PubMed
.
- S. H. Hong, A. Chlenov, M. W. Day and R. H. Grubbs, Angew. Chem., Int. Ed., 2007, 46, 5148–5151 CrossRef CAS PubMed
.
- A. Poater and L. Cavallo, J. Mol. Catal. A: Chem., 2010, 324, 75–79 CrossRef CAS PubMed
.
- A. Poater, N. Bahri-Laleh and L. Cavallo, Chem. Commun., 2011, 47, 6674–6676 RSC
.
- J. Mathew, N. Koga and C. H. Suresh, Organometallics, 2008, 27, 4666–4670 CrossRef CAS
.
- K. Vehlow, S. Gessler and S. Blechert, Angew. Chem., Int. Ed., 2007, 46, 8082–8085 CrossRef CAS PubMed
.
- B. K. Keitz, K. Endo, M. B. Herbert and R. H. Grubbs, J. Am. Chem. Soc., 2011, 133, 9686–9688 CrossRef CAS PubMed
.
- M. B. Herbert, Y. Lan, B. K. Keitz, P. Liu, K. Endo, M. W. Day, K. N. Houk and R. H. Grubbs, J. Am. Chem. Soc., 2012, 134, 7861–7866 CrossRef CAS PubMed
.
- B. R. Galan, M. Gembicky, P. M. Dominiak, J. B. Keister and S. T. Diver, J. Am. Chem. Soc., 2005, 127, 15702–15703 CrossRef CAS PubMed
.
- B. R. Galan, M. Pitak, M. Gembicky, J. B. Keister and S. T. Diver, J. Am. Chem. Soc., 2009, 131, 6822–6832 CrossRef CAS PubMed
.
- B. R. Galan, K. P. Kalbarczyk, S. Szczepankiewicz, J. B. Keister and S. T. Diver, Org. Lett., 2007, 9, 1203–1206 CrossRef CAS PubMed
.
- A. Poater, F. Ragone, A. Correa and L. Cavallo, J. Am. Chem. Soc., 2009, 131, 9000–9006 CrossRef CAS PubMed
.
- B. Schmidt, J. Mol. Catal. A: Chem., 2006, 254, 53–57 CrossRef CAS PubMed
.
- B. Schmidt, Eur. J. Org. Chem., 2004, 1865–1880 CrossRef CAS
.
- S. Manzini, C. A. Urbina-Blanco, A. Poater, A. M. Z. Slawin, L. Cavallo and S. P. Nolan, Angew. Chem., 2012, 124, 1066–1069 CrossRef
.
- J. A. Fernández-Salas, S. Manzini and S. P. Nolan, Adv. Synth. Catal., 2014, 356, 308–312 CrossRef
.
- S. Manzini, D. J. Nelson, T. Lebl, A. Poater, L. Cavallo, A. M. Z. Slawin and S. P. Nolan, Chem. Commun., 2014, 50, 2205–2207 RSC
.
- S. Manzini, D. J. Nelson, A. Poater, L. Cavallo, A. M. Z. Slawin and S. P. Nolan, Angew. Chem., Int. Ed., 2014 DOI:10.1002/anie.201403770
.
- T. M. Trnka, J. P. Morgan, M. S. Sanford, T. E. Wilhelm, M. Scholl, T.-L. Choi, S. Ding, M. W. Day and R. H. Grubbs, J. Am. Chem. Soc., 2003, 125, 2546–2558 CrossRef CAS PubMed
.
- W. J. van Rensburg, P. J. Steynberg, W. H. Meyer, M. M. Kirk and G. S. Forman, J. Am. Chem. Soc., 2004, 126, 14332–14333 CrossRef CAS PubMed
.
- W. J. van Rensburg, P. J. Steynberg, M. M. Kirk, W. H. Meyer and G. S. Forman, J. Organomet. Chem., 2006, 691, 5312–5325 CrossRef CAS PubMed
.
Footnotes |
| † Current address: WestCHEM Department of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, G1 1XL, UK. |
| ‡ Current address: Lehrstuhl für Technische Chemie und Petrolchemie, Institut für Technische und Makromolekulare Chemie (ITMC), RWTH Aachen University, Worringerweg 1, 52074 Aachen, Germany. |
| This journal is © The Royal Society of Chemistry 2014 |